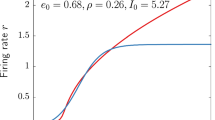
Abstract
Neural mass models (NMMs) are efficient frameworks for describing macroscopic cortical dynamics including electroencephalogram and magnetoencephalogram signals. Originally, these models were formulated on an empirical basis of synaptic dynamics with relatively long time constants. By clarifying the relations between NMMs and the dynamics of microscopic structures such as neurons and synapses, we can better understand cortical and neural mechanisms from a multi-scale perspective. In a previous study, the NMMs were analytically derived by averaging the equations of synaptic dynamics over the neurons in the population and further averaging the equations of the membrane-potential dynamics. However, the averaging of synaptic current assumes that the neuron membrane potentials are nearly time invariant and that they remain at sub-threshold levels to retain the conductance-based model. This approximation limits the NMM to the non-firing state. In the present study, we newly propose a derivation of a NMM by alternatively approximating the synaptic current which is assumed to be independent of the membrane potential, thus adopting a current-based model. Our proposed model releases the constraint of the nearly constant membrane potential. We confirm that the obtained model is reducible to the previous model in the non-firing situation and that it reproduces the temporal mean values and relative power spectrum densities of the average membrane potentials for the spiking neurons. It is further ensured that the existing NMM properly models the averaged dynamics over individual neurons even if they are spiking in the populations.







Similar content being viewed by others
References
Barbieri F, Mazzoni A, Logothetis NK, Panzeri S, Brunel N (2014) Stimulus dependence of local field potential spectra: experiment versus theory. J Neurosci 34(44):14589–14605. doi:10.1523/JNEUROSCI.5365-13.2014
beim Graben P, Rodrigues S (2013) A biophysical observation model for field potentials of networks of leaky integrate-and-fire neurons. Front Comput Neurosci 6:100. doi:10.3389/fncom.2012.00100
Brunel N, Wang XJ (2003) What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol 90(1):415–430. doi:10.1152/jn.01095.2002
Cavallari S, Panzeri S, Mazzoni A (2014) Comparison of the dynamics of neural interactions between current-based and conductance-based integrate-and-fire recurrent networks. Front Neural Circuits 8:12. doi:10.3389/fncir.2014.00012
David O, Friston KJ (2003) A neural mass model for MEG/EEG: coupling and neuronal dynamics. Neuroimage 20:1743–1755. doi:10.1016/j.neuroimage.2003.07.015
David O, Cosmelli D, Friston KJ (2004) Evaluation of different measures of functional connectivity using a neural mass model. Neuroimage 21:659–673. doi:10.1016/j.neuroimage.2003.10.006
David O, Harrison L, Friston KJ (2005) Modelling event-related responses in the brain. Neuroimage 25:756–770. doi:10.1016/j.neuroimage.2004.12.030
Deco G, Jirsa VK, Robinson PA, Breakspear M, Friston K (2008) The dynamic brain: from spiking neurons to neural masses and cortical fields. PLoS Comput Biol 4:e1000092. doi:10.1371/journal.pcbi.1000092
Faugeras O, Touboul J, Cessac B (2009) A constructive mean-field analysis of multi-population neural networks with random synaptic weights and stochastic inputs. Front Comput Neurosci 3:1. doi:10.3389/neuro.10.001.2009
Freeman WJ (1975) Mass action in the nervous system. Academic Press, New York
Gerstner W (2000) Population dynamics of spiking neurons: fast transients, asynchronous states, and locking. Neural Comput 12:43–89. doi:10.1162/089976600300015899
Izhikevich EM, Edelman GM (2008) Large-scale model of mammalian thalamocortical systems. Proc Natl Acad Sci USA 105:3593–3598. doi:10.1073/pnas.0712231105
Jansen BH, Rit VG (1995) Electroencephalogram and visual evoked potential generation in a mathematical model of coupled cortical columns. Biol Cybern 73:357–366. doi:10.1007/BF00199471
Jansen BH, Zouridakis G, Brandt ME (1993) A neurophysiologically-based mathematical model of flash visual evoked potentials. Biol Cybern 68:275–283. doi:10.1007/BF00224863
Lopes da Silva FH, Hoeks A, Smits H, Zetterberg LH (1974) Model of brain rhythmic activity—the alpha-rhythm of the thalamus. Kybernetik 15:27–37
Mazzoni A, Panzeri S, Logothetis NK, Brunel N (2008) Encoding of naturalistic stimuli by local field potential spectra in networks of excitatory and inhibitory neurons. PLoS Comput Biol 4:e1000239. doi:10.1371/journal.pcbi.1000239
Mazzoni A, Whittingstall K, Brunel N, Logothetis NK, Panzeri S (2010) Understanding the relationships between spike rate and delta/gamma frequency bands of LFPs and EEGs using a local cortical network model. Neuroimage 52:956–972. doi:10.1016/j.neuroimage.2009.12.040
Moran R, Pinotsis DA, Friston K (2013) Neural masses and fields in dynamic causal modeling. Front Comput Neurosci 7:57. doi:10.3389/fncom.2013.00057
Moreno-Bote R, Renart A, Parga N (2008) Theory of input spike auto- and cross-correlations and their effect on the response of spiking neurons. Neural Comput 20:1651–1705. doi:10.1162/neco.2008.03-07-497
Naruse Y, Matani A, Miyawaki Y, Okada M (2010) Influence of coherence between multiple cortical columns on alpha rhythm: a computational modeling study. Hum Brain Mapp 31:703–715. doi:10.1002/hbm.20899
Richardson MJE (2004) Effects of synaptic conductance on the voltage distribution and firing rate of spiking neurons. Phys Rev E 69:051918. doi:10.1103/PhysRevE.69.051918
Richardson MJE (2007) Firing-rate response of linear and nonlinear integrate-and-fire neurons to modulated current-based and conductance-based synaptic drive. Phys Rev E 76:021919. doi:10.1103/PhysRevE.76.021919
Robinson PA, Rennie CJ, Wright JJ, Bahramali H, Gordon E, Rowe DL (2001) Prediction of electroencephalographic spectra from neurophysiology. Phys Rev E 63:021903. doi:10.1103/PhysRevE.63.021903
Robinson PA, Rennie CJ, Rowe DL, O’Connor SC (2004) Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Hum Brain Mapp 23:53–72. doi:10.1002/hbm.20032
Robinson PA (2005) Propagator theory of brain dynamics. Phys Rev E 72:011904. doi:10.1103/PhysRevE.72.011904
Rodrigues S, Chizhov AV, Marten F, Terry JR (2010) Mappings between a macroscopic neural-mass model and a reduced conductance-based model. Biol Cybern 102:361–371. doi:10.1007/s00422-010-0372-z
Saito Y, Mitsui T (1993) Simulation of stochastic differential equations. Ann Inst Stat Math 45:419–432. doi:10.1007/BF00773344
Teramae J, Fukai T (2007) Local cortical circuit model inferred from power-law distributed neuronal avalanches. J Comput Neurosci 22:301–302. doi:10.1007/s10827-006-0014-6
Teramae J, Tsubo Y, Fukai T (2012) Optimal spike-based communication in excitable networks with strong-sparse and weak-dense links. Sci Rep 2:485. doi:10.1038/srep00485
van Hemmen JL (2004) Continuum limit of discrete neuronal structures: is cortical tissue an gexcitableh medium? Biol Cybern 91:347–358. doi:10.1007/s00422-004-0530-2
Wendling F, Bellanger JJ, Bartolomei F, Chauvel P (2000) Relevance of nonlinear lumped-parameter models in the analysis of depth-EEG epileptic signals. Biol Cybern 83:367–378. doi:10.1007/s004220000160
Zandt BJ, Visser S, van Putten MJAM, ten Haken B (2014) A neural mass model based on single cell dynamics to model pathophysiology. J Comput Neurosci 37:549–568. doi:10.1007/s10827-014-0517-5
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 26330293.
Author information
Authors and Affiliations
Corresponding author
Appendix: Input power and cross spectra
Appendix: Input power and cross spectra
This appendix derives Eq. (35), which computes the power and cross-spectrum densities of the input noises \(\tilde{\phi }_{{\mathrm X}_{\mathrm E}}(t)\) and \(\tilde{\phi }_{{\mathrm X}_{\mathrm I}}(t)\) imposed on the excitatory and inhibitory populations, respectively. Every Poisson spike train \(\zeta _i(t)\) in Eq. (29), firing at the rate given by Eq. (30), matches the definition of Mazzoni et al. (2008), beim Graben and Rodrigues (2013), and Cavallari et al. (2014).
The Poisson spike train of a neuron is approximated by white Gaussian noise, which is averaged over the neurons of a population. The ensemble mean of the inhomogeneous Poisson process \(\zeta _i(t)\) with time-varying rate \(\rho (t)=\rho _0+\eta (t)\) for the i-th neuron is expressed as
where \(\xi _i(t)\) is white Gaussian noise with zero mean and unit variance (Moreno-Bote et al. 2008). As the white Gaussian noise \(\xi _i(t)\) is orthogonal to the probability process \(\eta (t)\) in Eq. (30), we can compute the ensemble mean, representing white Gaussian noise with a time-varying mean of \(\rho _0+\eta (t)\) and variance of \(\rho _0\):
where averaging over the neurons in the population \(\mu \) decreases the variance by a factor of \(1/N_\mu \) for \(\mu \in \{{\mathrm E},{\mathrm I}\}\). Accordingly, the standard deviation \(\sqrt{\rho _0}\), i.e., a coefficient of \(\left\langle {\xi _i(t)}\right\rangle \), decreases to \(\sqrt{\rho _0/N_\mu }\). The average noise of the population \(\mu \) is given by
where \(\xi _\mu (t)\) is white Gaussian noise with zero mean and unit variance such that this noise is independent of \(\xi _i(t)\) for any i. Therefore, the correlation function between the average noises for the Poisson processes of neuron populations \(\mu \) and \(\nu \), \(\{\mu ,\nu \}\in \{{\mathrm {E}}, {\mathrm {I}}\}\), respectively, is given by
The spectrum density is straightforwardly solved as the Fourier transform of the correlation function:
The result is Eq. (35). Note that the final term,
is the PSD of the OU process \(\eta (t)\).
Finally, we estimate the order of magnitude of each term in Eq. (35). Except at extremely high frequencies, the first term is negligibly small even in the nonzero case \(\mu =\nu \). In the network model of neurons (as in Mazzoni et al. 2008), we set \(\rho _0 \sim 1\) spikes/ms, \(\epsilon _{\mathrm X}\sim 10\) ms, \(\sigma _{\mathrm X}^2\sim 0.1\) spikes/ms, and \(N_{\mathrm I}\sim 1000\). At frequencies below 100 Hz, i.e., \(\rho _0/(2\pi {N_\mu })<0.1\) kHz, the orders of magnitude of the respective terms are
Therefore, in this frequency band, the first term is approximately 1000 times smaller than the other terms in Eq. (35), and accordingly, the PSD was approximately obtained as Eq. (36).
Rights and permissions
About this article
Cite this article
Umehara, H., Okada, M., Teramae, Jn. et al. Macroscopic neural mass model constructed from a current-based network model of spiking neurons. Biol Cybern 111, 91–103 (2017). https://doi.org/10.1007/s00422-017-0710-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-017-0710-5