Abstract
A salamander is an ideal animal for studying the spinal locomotor network mechanism of vertebrates from an evolutionary perspective since it represents the transition from an aquatic to a terrestrial animal. However, little is known about the spinal locomotor network of a salamander. A spinal locomotor network model is a useful tool for exploring the working mechanism of the spinal networks of salamanders. A new spinal locomotor network model for a salamander is built for a three-dimensional (3D) biomechanical model of the salamander using a novel locomotion-controlled neural network model. Based on recent experimental data on the spinal circuitry and observational results of gaits of vertebrates, we assume that different interneuron sets recruited for mediating the frequency of spinal circuits are also related to the generation of different gaits. The spinal locomotor networks of salamanders are divided into low-frequency networks for walking and high-frequency networks for swimming. Additionally, a new topological structure between the body networks and limb networks is built, which only uses the body networks to coordinate the motion of limbs. There are no direct synaptic connections among limb networks. These techniques differ from existing salamander spinal locomotor network models. A simulation is performed and analyzed to validate the properties of the new spinal locomotor networks of salamanders. The simulation results show that the new spinal locomotor networks can generate a forward walking gait, a backward walking gait, a swimming gait, and a turning gait during swimming and walking. These gaits can be switched smoothly by changing external inputs from the brainstem. These properties are consistent with those of a real salamander. However, it is still difficult for the new spinal locomotor networks to generate highly efficient turning during walking, 3D swimming, nonrhythmic movements, and so on. New experimental data are required for further validation.








Similar content being viewed by others
References
Ampatzis K, Song J, Ausborn J, Manira AEI (2013) Pattern of innervation and recruitment of different classes of motoneurons in adult zebrafish. J Neurosci 33(26):10875–10886
Ampatzis K, Song J, Ausborn J, Manira AEI (2014) Separate microcircuit modules of distinct V2a interneurons and motoneurons control the speed of locomotion. Neuron 83(4):934–943
Ashley-Ross MA (1994) Hindlimb kinematics during terrestrial locomotion in a salamander (Dicamptodon tenebrosus). J Exp Biol 193(1):255–283
Ashley-Ross MA, Lauder GV (1997) Motor patterns and kinematics during backward walking in the pacific giant salamander: evidence for novel motor output. J Neurophysiol 78:3847–3860
Ausborn J, Mahmood R, Manira AEI (2012) Decoding the rules of recruitment of excitatory interneurons in the adult zebrafish locomotor network. Proc Nati Acad Sci 109:3631–3639
Bem T, Cagelguen J-M, Ekeberg Ö, Grillner S (2003) From swimming to walking: a single basic network for two different behaviors. Biol Cybern 88(2):79–90
Bicanski A, Ryczko D, Knuesel J, Harischandra N, Charrier V, Ekegerg Ö, Cabelguen J-M, Ijspeert AJ (2013a) Decoding the mechanisms of gait generation in salamanders by combining neurobiology, modeling and robotics. Biol Cybern 107(5):545–564
Bicanski A, Ryczko D, Cabelguen J-M, Ijspeert AJ (2013b) From lamprey to salamander: an exploratory modeling study on the architecture of the spinal locomotor networks in the salamander. Biol Cybern 107(5):565–587
Bolt JR (1969) Lisamphibians origins: possible protolissamphibian from the Lower Permian of Oklahoma. Science 166:888–891
Bolt JR (1977) Dissorophoid relationships and ontogeny and the origin of Lissamphibia. J Paleontol 51:235–249
Chevallier S, Ijspeert AJ, Ryczko D, Nagy F, Cabelguen J-M (2008) Organization of the spinal central pattern generators for locomotion in the salamander: biology and modeling. Brain Res Rev S7:147–161
Delvolve I, Bem T, Cabelguen J-M (1997) Epaxial and limb muscle activity during swimming and terrestrial stepping in the adult newt, Pleurodeles waltl. J Neurophysiol 78(2):638–650
Ekeberg Ö (1993) A combined neuronal and mechanical model of fish swimming. Biol Cybern 69(5):363–374
Green MH, Hale ME (2012) Activity of pectoral fin motoneurons during two swimming gaits in the larval zebrafish (Danio rerio) and localization of upstream circuit elements. J Neurophysiol 108:3393–3402
Grillner S, Ekeberg Ö, Manira AE, Lansner A, Parker D, Tegnér J, Wallén P (1998) Intrinsic function of a neuronal network—a vertebrate central pattern generator. Brain Res Rev 26(4):184–197
Guertin PA (2009) The mammalian central pattern generator for locomotion. Brain Res Rev 62:45–56
Hale ME, Day RD, Thorsen DH, Westneat MW (2006) Pectoral fin coordination and gait transitions in steadily swimming juvenile reef fishes. J Exp Biol 209:3708–3718
Harischandra N, Cabelguen J-M, Ekeberg Ö (2010) A 3D musculo-mechanical model of the salamander for the study of different gaits and modes of locomotion. Front Neurorobot 4:1–10
Harischandra N, Knuesel J, Kozlov A, Bicanski A, Cabelguen J-M, Ijspeert A, Ekeberg Ö (2011) Sensory feedback plays a significant role in generating walking gait and in gait transition in salamanders: a simulation study. Front Neurorobot 5:1–13
Ijspeert AJ (2000) A 3-D biomechanical model of the salamander. Lect Notes Comput Sci 1834:225–234
Ijspeert AJ (2001) A connectionist central pattern generator for the aquatic and terrestrial gaits of a simulated salamander. Biol Cybern 84(5):331–348
Ijspeert AJ (2008) Central pattern generators for locomotion control in animals and robots: a review. Neural Netw 21(4):642–653
Ijspeert AJ, Crespi A, Ryczko D, Cabelguen J-M (2007) From swimming to walking with a salamander robot driven by a spinal cord model. Science 315(3):1416–1420
Karakasiliotis K, Thandiackal R, Melo K, Horvat T, Mahabadi NK, Tsitkov S, Cabelguen JM, Ijspeert AJ (2016) From cineradiography to biorobots: an approach for designing robots to emulate and study animal locomotion. J R Soc Interface 13(119):1–15
Knüsel J, Bicanski A, Ryczko D, Cabelguen J-M, Ijspeert AJ (2013) A salamander’s flexible spinal network for locomotion, modeled at two levels of abstraction. Integr Comp Biol 53(3):1–14
Kozlov A, Huss M, Lansner A et al (2009) Simple cellular and network control principles govern complex patterns of motor behavior. Proc Nati Acad Sci 106:20027–20032
Liu Q, Wang JZ (2018) Modeling and analysis of a new locomotion control neural network. Biol Cybern. https://doi.org/10.1007/s00422-018-0758-x
McLean DL, Fetcho JR (2009) Spinal interneurons differentiate sequentially from those driving the fastest swimming movements in larval zebrafish to those driving the slowest ones. J Neurosci 29(43):13566–13577
McLean D, Fan J, Higasshijima S, Hale ME, Fetcho JR (2007) A topographic map of recruitment in spinal cord. Nature 446(3):71–75
McLean DL, Masino MA, Koh IYY, Lindquist WB, Fetcho JR (2008) Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci 11(12):1419–1429
Menelaou E, McLean DL (2013) Speed control: spinal interneurons with crossed purposes. Curr Biol 23(17):716–718
Menelaou E, VanDunk C, McLean DL (2014) Differences in the morphology of spinal V2a neurons reflect their recruitment order during swimming in larval zebrafish. J Comp Neurol 522(6):1232–1248
Ryczko D, Knüsel J, Crespi A, Lamarque S, Mathou A, Ijspeert AJ, Cabelguen JM (2015) Flexibility of the axial central pattern generator network for locomotion in the salamander. J Neurophysiol 113:1921–1940
Wallén P, Grillner S (1987) N-methyl-D-aspartate receptor-induced, inherent oscillatory activity in neurons active during fictive locomotion in the lamprey. J Neurosci 7(9):2745–2755
Acknowledgements
This work is supported in part by the National Natural Science Foundation of China under Grant 61105110 and 11573011, the Natural Science Foundation of the Jiangsu Higher Education Institutions of China under Grant 14KJB510004, the Jiangsu Overseas Research and Training Program for University Prominent Young and Middle-aged Teachers and President, the Lianyungang “521” Project, and the Six Talent Peaks Project in Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Benjamin Lindner.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
1.1 A: Detailed description of the biomechanical model of a salamander
For the new biomechanical model of a salamander, built using the ADAMS software, a virtual entity model needs to be built and the constraints added based on Fig. 1. The forces from the water and muscles and the contact forces between the links can then be applied to the virtual entity model of a salamander.
Parameters of virtual entity model The virtual model of a salamander has ten links for the body and eight links for the limbs. Their parameters are shown in Table 4.
Forces due to water Neglecting the fluid forces of limbs, the fluid forces \(F_i ,(i=1,\ldots ,10)\) of the links of the body can be calculated as
where \(\rho \) is the density of the water; \(l_i \) is the length of the link i of the body; \(h_i \) is the height of the link i of the body; \(C_i \) is the drag coefficient of the link i of the body (here \(C_i =1\) for all links of the body); \(v_i \) is the normal velocity of the link i at the midpoint of the body; and p is the switch variable between walking and swimming (during walking \(p=0\) and during swimming).
Torque of muscles A muscle can be simulated using a combination of a spring and a damper (Ekeberg 1993). Using the muscle model as in the reference, the torque acting on the joint of a salamander can be calculated as
where \(M_f \) are the motoneuron activities of the flexor muscles; \(M_e \) are the motoneuron activities of the extensor muscles; \(\Delta \varphi \) is the difference between the actual angle of the joint and the resting angle; and \(\alpha ,\beta \),\(\xi \), and \(\delta \) indicate respectively the gain, stiffness gain, tonic stiffness, and damping coefficient of the muscles. The parameters of the muscles are shown in Table 5.
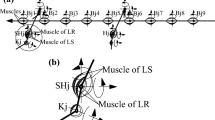
Constraint and contact for biomechanical model of salamander Based on the moving range of the shoulder joints and hip joints, constraint blocks surrounding the thighs are added, and the contact between each constraint block and the thighs is added. Based on the moving range of the body joints and the elbow (or knee) joints, the constraint blocks located between the body joints and between the thighs and the shanks, as well as the contact between them, are also added. In addition, the contacts between the biomechanical model of a salamander and the ground are added (see Sect. 2 for further details).
1.2 B: Detailed description of spinal locomotor networks of a salamander
Parameters of spinal locomotor networks During gait generation and gait transition, all parameters of the spinal locomotor networks remain unchanged, except for \(\tau _i \) and \(\gamma _i \). Both of these are used to control the frequency of the spinal locomotor networks. It is assumed here that the parameters of all oscillators are the same, and all neurons have a rhythmic output under default conditions. Based on Liu and Wang (2018), we choose the parameters of the oscillator to be \(\tau _i ={0.5},\gamma _i =0.3,\varepsilon _i =10,\sigma _i =1,a_{ii} =14,b_i =8, \theta _i =0,\bar{{x}}_i ={1}0(i=1,\ldots ,62), a_{ii+1} =-0.8,a_{i+1i} =-0.6(i=1,3,\ldots ,61)\). The excitatory weight between the oscillators of the body is 0.4. The excitatory weight from the oscillators of the body to the oscillators of the limbs is 0.9. The excitatory weight from the oscillators of the limbs to the oscillators of the body is 0.9. The inhibitory weight between the oscillators of the body and the oscillators of the limbs is \(-0.9\). The excitatory weight between the oscillators of the limbs is 0.9.
Range of external inputs Based on Liu and Wang (2018), the tonic external inputs of the networks, which make the networks generate rhythmic outputs or become inactive or saturated, are related to the topology of the networks. Since the topology of the networks for forward walking, backward walking, and swimming are different, we calculate their respective tonic external inputs based on Liu and Wang (2018). When only neuron i is inactive or saturated and the other neurons generate rhythmic outputs, the upper bound of inactivity and the lower bound of saturation of the tonic external inputs \(s_i \) for the forward walking and backward walking networks are as shown in Tables 6 and 7, respectively. The corresponding results for the swimming networks are shown in Table 8.
Based on Tables 6, 7, and 8, it can be seen that the output of a neuron is inactive, saturated, or rhythmic when the tonic external input is less than the upper bound of inactivity, greater than the lower bound of saturation, or between them, respectively.
Initial states of spinal locomotor networks The initial states are helpful for the transition performance of spinal locomotor networks. Based on the biological properties of a real neuron, the initial states of the spinal locomotor networks are calculated. Since the resting potential of a neuron is lower than the firing threshold, the resting potential \(x_{i0} ,(i=1,\ldots ,62)\) is chosen to be −0.1. In addition, during the resting stage, we assume the change rate of the membrane potential of the neuron is \(\dot{x}_i =0,(i=1,\ldots ,62)\). Based on Eq. (1), the initial states of the spinal locomotor networks can be calculated as follows:
Rights and permissions
About this article
Cite this article
Liu, Q., Yang, H., Zhang, J. et al. A new model of the spinal locomotor networks of a salamander and its properties. Biol Cybern 112, 369–385 (2018). https://doi.org/10.1007/s00422-018-0759-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-018-0759-9