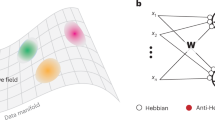
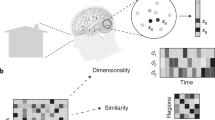
Abstract
The brain displays a remarkable ability to sustain stable memories, allowing animals to execute precise behaviors or recall stimulus associations years after they were first learned. Yet, recent long-term recording experiments have revealed that single-neuron representations continuously change over time, contravening the classical assumption that learned features remain static. How do unstable neural codes support robust perception, memories, and actions? Here, we review recent experimental evidence for such representational drift across brain areas, as well as dissections of its functional characteristics and underlying mechanisms. We emphasize theoretical proposals for how drift need not only be a form of noise for which the brain must compensate. Rather, it can emerge from computationally beneficial mechanisms in hierarchical networks performing robust probabilistic computations.



Similar content being viewed by others
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Adesnik H, Abdeladim L (2021) Probing neural codes with two-photon holographic optogenetics. Nat Neurosci pp 1–11. https://doi.org/10.1038/s41593-021-00902-9
Ahrens MB, Orger MB, Robson DN et al (2013) Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods 10(5):413–420. https://doi.org/10.1038/nmeth.2434
Aitchison L (2020) Why bigger is not always better: on finite and infinite neural networks. In: III HD, Singh A (eds) Proceedings of the 37th International Conference on Machine Learning, Proceedings of Machine Learning Research, vol 119. PMLR, pp 156–164
Aitchison L, Lengyel M (2017) With or without you: predictive coding and Bayesian inference in the brain. Curr Opin Neurobiol 46:219–227. https://doi.org/10.1016/j.conb.2017.08.010
Aitchison L, Jegminat J, Menendez JA et al (2021) Synaptic plasticity as Bayesian inference. Nat Neurosci 24(4):565–571. https://doi.org/10.1038/s41593-021-00809-5
Amit DJ, Fusi S (1994) Learning in neural networks with material synapses. Neural Comput 6(5):957–982. https://doi.org/10.1162/neco.1994.6.5.957
de Andrade Costa A, Copelli M, Kinouchi O (2015) Can dynamical synapses produce true self-organized criticality? Journal of Statistical Mechanics: Theory and Experiment 2015(6):P06004. https://doi.org/10.1088/1742-5468/2015/06/P06004
Angelucci A, Levitt JB, Walton EJ et al (2002) Circuits for local and global signal integration in primary visual cortex. J Neurosci 22(19):8633–8646. https://doi.org/10.1371/journal.pcbi.1005582
Attardo A, Fitzgerald JE, Schnitzer MJ (2015) Impermanence of dendritic spines in live adult CA1 hippocampus. Nature 523(7562):592–596. https://doi.org/10.1038/nature14467
Baldi P, Hornik K (1989) Neural networks and principal component analysis: Learning from examples without local minima. Neural Netw 2(1):53–58. https://doi.org/10.1016/0893-6080(89)90014-2
Banerjee A, Egger R, Long MA (2021) Using focal cooling to link neural dynamics and behavior. Neuron. https://doi.org/10.1016/j.neuron.2021.05.029
Barnes CA, Suster MS, Shen J et al (1997) Multistability of cognitive maps in the hippocampus of old rats. Nature 388(6639):272–275. https://doi.org/10.1038/40859
Barrett DG, Deneve S, Machens CK (2016) Optimal compensation for neuron loss. eLife 5(e12):454. https://doi.org/10.7554/eLife.12454
Beggs JM, Timme N (2012) Being critical of criticality in the brain. Front Physiol 3:163. https://doi.org/10.3389/fphys.2012.00163
Buesing L, Bill J, Nessler B et al (2011) Neural dynamics as sampling: a model for stochastic computation in recurrent networks of spiking neurons. PLoS Comput Biol. https://doi.org/10.1371/journal.pcbi.1002211
Calaim N, Dehmelt FA, Gonçalves PJ, et al. (2020) Robust coding with spiking networks: a geometric perspective. bioRxiv https://doi.org/10.1101/2020.06.15.148338
Carandini M, Heeger DJ (2012) Normalization as a canonical neural computation. Nat Rev Neurosci 13(1):51–62. https://doi.org/10.1038/nrn3136
Chalk M, Masset P, Deneve S et al (2017) Sensory noise predicts divisive reshaping of receptive fields. PLoS Comput Biol. https://doi.org/10.1371/journal.pcbi.1005582
Chalk M, Tkacik G, Marre O (2021) Inferring the function performed by a recurrent neural network. Plos ONE. https://doi.org/10.1371/journal.pone.0248940
Chestek CA, Batista AP, Santhanam G et al (2007) Single-neuron stability during repeated reaching in macaque premotor cortex. J Neurosci 27(40):10742–10750. https://doi.org/10.1523/JNEUROSCI.0959-07.2007
Chung JE, Magland JF, Barnett AH et al (2017) A fully automated approach to spike sorting. Neuron 95(6):1381-1394 https://doi.org/10.1016/j.neuron.2017.08.030
Chung JE, Joo HR, Fan JL et al (2019) High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 101(1):21–31. https://doi.org/10.1016/j.neuron.2018.11.002
Clopath C, Bonhoeffer T, Hübener M et al (2017) Variance and invariance of neuronal long-term representations. Philos Trans R Soc B: Biol Sci 372(1715):20160161. https://doi.org/10.1098/rstb.2016.0161
Das A, Levina A (2019) Critical neuronal models with relaxed timescale separation. Phys Rev X. https://doi.org/10.1103/PhysRevX.9.021062
Deitch D, Rubin A, Ziv Y (2021) Representational drift in the mouse visual cortex. Curr Biol. https://doi.org/10.1016/j.cub.2021.07.062
Denève S, Alemi A, Bourdoukan R (2017) The brain as an efficient and robust adaptive learner. Neuron 94(5):969–977. https://doi.org/10.1016/j.neuron.2017.05.016
Dhawale AK, Poddar R, Wolff SB, et al. (2017) Automated long-term recording and analysis of neural activity in behaving animals. eLife 6:e27702 https://doi.org/10.7554/eLife.27702
Dickey AS, Suminski A, Amit Y et al (2009) Single-unit stability using chronically implanted multielectrode arrays. J Neurophysiol 102(2):1331–1339. https://doi.org/10.1152/jn.90920.2008
Dimitriadis G, Neto JP, Aarts A, et al. (2018) Why not record from every channel with a CMOS scanning probe? bioRxiv https://doi.org/10.1101/275818
Driscoll LN, Pettit NL, Minderer M et al (2017) Dynamic reorganization of neuronal activity patterns in parietal cortex. Cell 170(5):986–999. https://doi.org/10.1016/j.cell.2017.07.021
Ebitz RB, Hayden BY (2021) The population doctrine in cognitive neuroscience. Neuron. https://doi.org/10.1016/j.neuron.2021.07.011
Echeveste R, Aitchison L, Hennequin G et al (2020) Cortical-like dynamics in recurrent circuits optimized for sampling-based probabilistic inference. Nat Neurosci 23(9):1138–1149. https://doi.org/10.1038/s41593-020-0671-1
Fiser J, Berkes P, Orbán G et al (2010) Statistically optimal perception and learning: from behavior to neural representations. Trends Cogn Sci 14(3):119–130. https://doi.org/10.1016/j.tics.2010.01.003
French RM (1999) Catastrophic forgetting in connectionist networks. Trends Cogn Sci 3(4):128–135. https://doi.org/10.1016/s1364-6613(99)01294-2
Fusi S (2021) Memory capacity of neural network models. arXiv preprint arXiv:2108.07839
Fusi S, Abbott L (2007) Limits on the memory storage capacity of bounded synapses. Nat Neurosci 10(4):485–493. https://doi.org/10.1038/nn1859
Fusi S, Senn W (2006) Eluding oblivion with smart stochastic selection of synaptic updates. Chaos: An Interdisciplinary Journal of Nonlinear Science 16(2):026112. https://doi.org/10.1063/1.2213587
Gallego JA, Perich MG, Miller LE et al (2017) Neural manifolds for the control of movement. Neuron 94(5):978–984. https://doi.org/10.1016/j.neuron.2017.05.025
Gallego JA, Perich MG, Naufel SN et al (2018) Cortical population activity within a preserved neural manifold underlies multiple motor behaviors. Nat Commun 9(1):4233. https://doi.org/10.1038/s41467-018-06560-z
Gallego JA, Perich MG, Chowdhury RH et al (2020) Long-term stability of cortical population dynamics underlying consistent behavior. Nat Neurosci 23(2):260–270. https://doi.org/10.1038/s41593-019-0555-4
Gao P, Trautmann E, Yu B, et al (2017) A theory of multineuronal dimensionality, dynamics and measurement. bioRxiv p 214262. https://doi.org/10.1101/214262
Gardiner CW (1985) Handbook of stochastic methods, vol 3. Springer, Berlin
Geiger M, Spigler S, d’Ascoli S et al (2019) Jamming transition as a paradigm to understand the loss landscape of deep neural networks. Phys Rev E 100(1):012115. https://doi.org/10.1103/PhysRevE.100.012115
Giovannucci A, Friedrich J, Gunn P, et al (2019) Caiman an open source tool for scalable calcium imaging data analysis. eLife 8:e38173. https://doi.org/10.7554/eLife.38173
Glaze CM, Troyer TW (2006) Temporal structure in zebra finch song: implications for motor coding. J Neurosci 26(3):991–1005. https://doi.org/10.1523/JNEUROSCI.3387-05.2006
Goaillard JM, Marder E (2021) Ion channel degeneracy, variability, and covariation in neuron and circuit resilience. Annual Review of Neuroscience 44. https://doi.org/10.1146/annurev-neuro-092920-121538
Goldt S, Krzakala F, Zdeborová L, et al (2021) Bayesian reconstruction of memories stored in neural networks from their connectivity. arXiv preprint arXiv:2105.07416
Gonzalez WG, Zhang H, Harutyunyan A et al (2019) Persistence of neuronal representations through time and damage in the hippocampus. Science 365(6455):821–825. https://doi.org/10.1126/science.aav9199
Hahnloser RH, Kozhevnikov AA, Fee MS (2002) An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419(6902):65–70. https://doi.org/10.1038/nature00974
Harvey CD, Coen P, Tank DW (2012) Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484(7392):62–68. https://doi.org/10.1038/nature10918
Hiratani N, Fukai T (2018) Redundancy in synaptic connections enables neurons to learn optimally. Proc Natl Acad Sci 115(29):E6871–E6879. https://doi.org/10.1073/pnas.1803274115
Hubel D (1995) Eye, Brain, and Vision. Scientific American Library series, Henry Holt and Company
Inagaki HK, Fontolan L, Romani S et al (2019) Discrete attractor dynamics underlies persistent activity in the frontal cortex. Nature 566(7743):212–217. https://doi.org/10.1038/s41586-019-0919-7
Izmailov P, Vikram S, Hoffman MD, et al (2021) What are Bayesian neural network posteriors really like? In: Meila M, Zhang T (eds) Proceedings of the 38th International Conference on Machine Learning, Proceedings of Machine Learning Research, vol 139. PMLR, pp 4629–4640
Jazayeri M, Afraz A (2017) Navigating the neural space in search of the neural code. Neuron 93(5):1003–1014. https://doi.org/10.1016/j.neuron.2017.02.019
Jensen KT, Harpaz NK, Dhawale AK, et al (2021) Long-term stability of neural activity in the motor system. bioRxiv https://doi.org/10.1101/2021.10.27.465945
Juavinett AL, Bekheet G, Churchland AK (2019) Chronically implanted neuropixels probes enable high-yield recordings in freely moving mice. eLife 8. https://doi.org/10.7554/eLife.47188
Jun JJ, Mitelut C, Lai C, et al (2017) Real-time spike sorting platform for high-density extracellular probes with ground-truth validation and drift correction. bioRxiv https://doi.org/10.1101/101030
Jun JJ, Steinmetz NA, Siegle JH et al (2017) Fully integrated silicon probes for high-density recording of neural activity. Nature 551(7679):232–236. https://doi.org/10.1038/nature24636
Kappel D, Habenschuss S, Legenstein R et al (2015) Network plasticity as Bayesian inference. PLoS Comput Biol. https://doi.org/10.1371/journal.pcbi.1004485
Katlowitz KA, Picardo MA, Long MA (2018) Stable sequential activity underlying the maintenance of a precisely executed skilled behavior. Neuron 98(6):1133–1140. https://doi.org/10.1016/j.neuron.2018.05.017
Kaufman MT, Churchland MM, Ryu SI et al (2014) Cortical activity in the null space: permitting preparation without movement. Nat Neurosci 17(3):440–448. https://doi.org/10.1038/nn.3643
Kawaguchi K (2016) Deep learning without poor local minima. In: Lee D, Sugiyama M, Luxburg U et al (eds) Advances in Neural Information Processing Systems, vol 29. Curran Associates Inc
Kentros CG, Agnihotri NT, Streater S et al (2004) Increased attention to spatial context increases both place field stability and spatial memory. Neuron 42(2):283–295. https://doi.org/10.1016/s0896-6273(04)00192-8
Kirkpatrick J, Pascanu R, Rabinowitz N et al (2017) Overcoming catastrophic forgetting in neural networks. Proc Natl Acad Sci 114(13):3521–3526. https://doi.org/10.1073/pnas.1611835114
Kriegeskorte N, Wei XX (2021) Neural tuning and representational geometry. Nat Rev Neurosci. https://doi.org/10.1038/s41583-021-00502-3
Kulhavý R, Zarrop MB (1993) On a general concept of forgetting. Int J Control 58(4):905–924. https://doi.org/10.1080/00207179308923034
Kwon C, Ao P, Thouless DJ (2005) Structure of stochastic dynamics near fixed points. Proceed Natl Acad Sci 102(37):13029–13033. https://doi.org/10.1073/pnas.0506347102
Lee JS, Briguglio JJ, Cohen JD et al (2020) The statistical structure of the hippocampal code for space as a function of time, context, and value. Cell 183(3):620–635. https://doi.org/10.1016/j.cell.2020.09.024
Li M, Liu F, Jiang H et al (2017) Long-term two-photon imaging in awake macaque monkey. Neuron 93(5):1049–1057. https://doi.org/10.1016/j.neuron.2017.01.027
Li N, Daie K, Svoboda K et al (2016) Robust neuronal dynamics in premotor cortex during motor planning. Nature 532(7600):459–464. https://doi.org/10.1038/nature17643
Liberti WA, Markowitz JE, Perkins LN et al (2016) Unstable neurons underlie a stable learned behavior. Nat Neurosci 19(12):1665–1671. https://doi.org/10.1038/nn.4405
Llera-Montero M, Sacramento J, Costa RP (2019) Computational roles of plastic probabilistic synapses. Curr Opin Neurobiol 54:90–97. https://doi.org/10.1016/j.conb.2018.09.002
Long MA, Jin DZ, Fee MS (2010) Support for a synaptic chain model of neuronal sequence generation. Nature 468(7322):394–399. https://doi.org/10.1038/nature09514
Luo TZ, Bondy AG, Gupta D, et al (2020) An approach for long-term, multi-probe neuropixels recordings in unrestrained rats. eLife 9. https://doi.org/10.7554/eLife.59716
Mankin EA, Sparks FT, Slayyeh B et al (2012) Neuronal code for extended time in the hippocampus. Proceed Natl Acad Sci 109(47):19462–19467. https://doi.org/10.1073/pnas.1214107109
Marder E, Goeritz ML, Otopalik AG (2015) Robust circuit rhythms in small circuits arise from variable circuit components and mechanisms. Curr Opin Neurobiol 31:156–163. https://doi.org/10.1016/j.conb.2014.10.012
Marks TD, Goard MJ (2021) Stimulus-dependent representational drift in primary visual cortex. Nat Commun 12(1):5169. https://doi.org/10.1038/s41467-021-25825-8
Mau W, Hasselmo ME, Cai DJ (2020) The brain in motion: How ensemble fluidity drives memory-updating and flexibility. eLife 9:e63550. https://doi.org/10.7554/eLife.63550
Mongillo G, Rumpel S, Loewenstein Y (2017) Intrinsic volatility of synaptic connections–a challenge to the synaptic trace theory of memory. Curr Opin Neurobiol 46:7–13. https://doi.org/10.1016/j.conb.2017.06.006
Musk E, Neuralink, (2019) An integrated brain-machine interface platform with thousands of channels. J Med Internet Res. https://doi.org/10.2196/16194
Nayebi A, Srivastava S, Ganguli S, et al (2020) Identifying learning rules from neural network observables. arXiv preprint arXiv:2010.11765
Neal RM (1993) Bayesian learning via stochastic dynamics. In: Advances in Neural Information Processing Systems, pp 475–482
Øksendal B (2003) Stochastic differential equations. Springer, Berlin
Opper M (1999) A Bayesian approach to on-line learning. In: Saad D (ed) On-Line Learning in Neural Networks. Cambridge University Press, Publications of the Newton Institute, p 363-378, https://doi.org/10.1017/CBO9780511569920.017
Orbán G, Berkes P, Fiser J et al (2016) Neural variability and sampling-based probabilistic representations in the visual cortex. Neuron 92(2):530–543. https://doi.org/10.1016/j.neuron.2016.09.038
Pachitariu M, Steinmetz NA, Kadir S, et al (2016) Kilosort: realtime spike-sorting for extracellular electrophysiology with hundreds of channels. bioRxiv https://doi.org/10.1101/061481
Pachitariu M, Stringer C, Dipoppa M, et al (2017) Suite2p: beyond 10,000 neurons with standard two-photon microscopy. bioRxiv https://doi.org/10.1101/061507
Parisi G (1986) A memory which forgets. J Phys A: Math Gen 19(10):L617. https://doi.org/10.1088/0305-4470/19/10/011
Pashkovski SL, Iurilli G, Brann D et al (2020) Structure and flexibility in cortical representations of odour space. Nature 583(7815):253–258. https://doi.org/10.1038/s41586-020-2451-1
Pehlevan C, Sengupta AM, Chklovskii DB (2017) Why do similarity matching objectives lead to Hebbian/anti-Hebbian networks? Neural Comput 30(1):84–124. https://doi.org/10.1162/neco_a_01018
Pereira U, Brunel N (2018) Attractor dynamics in networks with learning rules inferred from in vivo data. Neuron 99(1):227–238. https://doi.org/10.1016/j.neuron.2018.05.038
Pérez-Ortega J, Alejandre-García T, Yuste R (2021) Long-term stability of cortical ensembles. eLife 10(e64):449. https://doi.org/10.7554/eLife.64449
Pnevmatikakis EA, Soudry D, Gao Y et al (2016) Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron 89(2):285–299. https://doi.org/10.1016/j.neuron.2015.11.037
Qin S, Farashahi S, Lipshutz D, et al (2021) Coordinated drift of receptive fields during noisy representation learning. bioRxiv https://doi.org/10.1101/2021.08.30.458264
Rao RP, Ballard DH (1999) Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci 2(1):79–87. https://doi.org/10.1038/4580
Rokni U, Richardson AG, Bizzi E et al (2007) Motor learning with unstable neural representations. Neuron 54(4):653–666. https://doi.org/10.1016/j.neuron.2007.04.030
Roland B, Deneux T, Franks KM, et al (2017) Odor identity coding by distributed ensembles of neurons in the mouse olfactory cortex. eLife 6:e26337. https://doi.org/10.7554/eLife.26337
Rule ME, O’Leary T (2021) Self-healing neural codes: Hebbian and homeostatic mechanisms can track evolving neural representations. bioRxiv https://doi.org/10.1101/2021.03.08.433413
Rule ME, O’Leary T, Harvey CD (2019) Causes and consequences of representational drift. Current Opinion in Neurobiology 58:141–147. https://doi.org/10.1016/j.conb.2019.08.005
Rule ME, Loback AR, Raman D, et al (2020) Stable task information from an unstable neural population. eLife 9:e51121. https://doi.org/10.7554/eLife.51121
Savin C, Deneve S (2014) Spatio-temporal representations of uncertainty in spiking neural networks. In: Advances in Neural Information Processing Systems, pp 2024–2032
Saxena S, Cunningham JP (2019) Towards the neural population doctrine. Curr Opin Neurobiol 55:103–111. https://doi.org/10.1016/j.conb.2019.02.002
Saxena S, Kinsella I, Musall S, et al (2020) Localized semi-nonnegative matrix factorization (LocaNMF) of widefield calcium imaging data. PLoS Computational Biology 16(4):e1007791. https://doi.org/10.1371/journal.pcbi.1007791
Schoonover CE, Ohashi SN, Axel R et al (2021) Representational drift in primary olfactory cortex. Nature. https://doi.org/10.1038/s41586-021-03628-7
Sengupta AM, Pehlevan C, Tepper M et al (2018) Manifold-tiling localized receptive fields are optimal in similarity-preserving neural networks. In: Bengio S, Wallach H, Larochelle H et al (eds) Advances in Neural Information Processing Systems, vol 31. Curran Associates Inc
Sheintuch L, Geva N, Baumer H et al (2020) Multiple maps of the same spatial context can stably coexist in the mouse hippocampus. Current Biology. https://doi.org/10.1016/j.cub.2020.02.018
Sofroniew NJ, Flickinger D, King J, et al (2016) A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. eLife 5:e14472. https://doi.org/10.7554/eLife.14472
Stavisky SD, Kao JC, Ryu SI et al (2017) Motor cortical visuomotor feedback activity is initially isolated from downstream targets in output-null neural state space dimensions. Neuron 95(1):195–208. https://doi.org/10.1016/j.neuron.2017.05.023
Steinmetz NA, Aydin C, Lebedeva A, et al (2021) Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. Science 372(6539):eabf4588. https://doi.org/10.1126/science.abf4588
Stettler DD, Axel R (2009) Representations of odor in the piriform cortex. Neuron 63(6):854–864. https://doi.org/10.1016/j.neuron.2009.09.005
Stevenson IH, Cherian A, London BM et al (2011) Statistical assessment of the stability of neural movement representations. J Neurophysiol 106(2):764–774. https://doi.org/10.1152/jn.00626.2010
Svoboda K, Yasuda R (2006) Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron 50(6):823–839. https://doi.org/10.1016/j.neuron.2006.05.019
Thompson L, Best P (1990) Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Res 509(2):299–308. https://doi.org/10.1016/0006-8993(90)90555-p
Tonegawa S, Pignatelli M, Roy DS et al (2015) Memory engram storage and retrieval. Curr Opin Neurobiol 35:101–109. https://doi.org/10.1016/j.conb.2015.07.009
Ulivi AF, Castello-Waldow TP, Weston G et al (2019) Longitudinal two-photon imaging of dorsal hippocampal CA1 in live mice. J Vis Exp 148(e59):598. https://doi.org/10.3791/59598
Urai AE, Doiron B, Leifer AM, et al (2021) Large-scale neural recordings call for new insights to link brain and behavior. arXiv preprint arXiv:210314662https://arxiv.org/abs/arXiv:2103.14662
Wanner AA, Friedrich RW (2020) Whitening of odor representations by the wiring diagram of the olfactory bulb. Nat Neurosci 23(3):433–442. https://doi.org/10.1038/s41593-019-0576-z
Welling M, Teh YW (2011) Bayesian learning via stochastic gradient Langevin dynamics. In: Proceedings of the 28th International Conference on Machine Learning, pp 681–688
Wilson AG, Izmailov P (2020) Bayesian deep learning and a probabilistic perspective of generalization. In: Larochelle H, Ranzato M, Hadsell R, et al (eds) Advances in Neural Information Processing Systems, vol 33. Curran Associates, Inc., pp 4697–4708
Xia J, Marks TD, Goard MJ et al (2021) Stable representation of a naturalistic movie emerges from episodic activity with gain variability. Nat Commun. https://doi.org/10.1038/s41467-021-25437-2
Yang G (2019) Scaling limits of wide neural networks with weight sharing. arXiv preprint arXiv:1902.04760
Yger P, Spampinato GL, Esposito E, et al (2018) A spike sorting toolbox for up to thousands of electrodes validated with ground truth recordings in vitro and in vivo. eLife 7. https://doi.org/10.7554/eLife.34518
Yu S, Ribeiro TL, Meisel C et al (2017) Maintained avalanche dynamics during task-induced changes of neuronal activity in nonhuman primates. Elife 6(e27):119. https://doi.org/10.7554/eLife.27119
Yuste R (2015) From the neuron doctrine to neural networks. Nat Rev Neurosci 16(8):487–497. https://doi.org/10.1038/nrn3962
Zavatone-Veth JA, Pehlevan C (2021) Exact marginal prior distributions of finite Bayesian neural networks. In: Ranzato M, Beygelzimer A, Liang P et al (eds) Advances in Neural Information Processing Systems, vol 34. Curran Associates Inc
Zavatone-Veth JA, Canatar A, Ruben BS et al (2021) Asymptotics of representation learning in finite Bayesian neural networks. In: Ranzato M, Beygelzimer A, Liang P et al (eds) Advances in Neural Information Processing Systems, vol 34. Curran Associates Inc
Zenke F, Poole B, Ganguli S (2017) Continual learning through synaptic intelligence. In: International Conference on Machine Learning, PMLR, pp 3987–3995
Zeraati R, Priesemann V, Levina A (2021) Self-organization toward criticality by synaptic plasticity. Front Phys 9:103. https://doi.org/10.3389/fphy.2021.619661
Ziv Y, Burns LD, Cocker ED et al (2013) Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci 16(3):264. https://doi.org/10.1038/nn.3329
Acknowledgements
We thank Cengiz Pehlevan and Venkatesh Murthy for support and mentorship. We also thank Matthew Farrell, Michael Goard, Siddharth Jayakumar, and Torben Ott for helpful comments on our manuscript.
Funding
PM was supported by a grant from the Harvard Mind Brain Behavior Interfaculty Initiative. SQ and JAZ-V were supported by the NIH (1UF1NS111697-01), the Intel Corporation (through the Intel Neuromorphic Research Community), and a Google Faculty Research Award. JAZ-V was also partially supported by the NSF-Simons Center for Mathematical and Statistical Analysis of Biology at Harvard, the Harvard Quantitative Biology Initiative, and the Harvard FAS Dean’s Competitive Fund for Promising Scholarship.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to conceptualization, literature review, and writing. They are listed alphabetically.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no other competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Jean-Marc Fellous.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors contributed equally and are listed alphabetically.
Rights and permissions
About this article
Cite this article
Masset, P., Qin, S. & Zavatone-Veth, J.A. Drifting neuronal representations: Bug or feature?. Biol Cybern 116, 253–266 (2022). https://doi.org/10.1007/s00422-021-00916-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-021-00916-3