Abstract
Stochastic resonance is known as a phenomenon whereby information transmission of weak signal or subthreshold stimuli can be enhanced by additive random noise with a suitable intensity. Another phenomenon induced by applying deterministic pulsatile electric stimuli with a pulse frequency, commonly used for deep brain stimulation (DBS), was also shown to improve signal-to-noise ratio in neuron models. The objective of this study was to test the hypothesis that pulsatile high-frequency stimulation could improve the detection of both sub- and suprathreshold synaptic stimuli by tuning the frequency of the stimulation in a population of pyramidal neuron models. Computer simulations showed that mutual information estimated from a population of neural spike trains displayed a typical resonance curve with a peak value of the pulse frequency at 80–120 Hz, similar to those utilized for DBS in clinical situations. It is concluded that a “pulse-frequency-dependent resonance” (PFDR) can enhance information transmission over a broad range of synaptically connected networks. Since the resonance frequency matches that used clinically, PFDR could contribute to the mechanism of the therapeutic effect of DBS.










Similar content being viewed by others
References
Ashkan K, Rogers P, Bergman H, Ughratdar I (2017) Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol 13:548–554
Axmacher N, Mormann F, Fernndez G, Elger C, Fell J (2006) Memory formation by neuronal synchronization. Brain Res Rev 52:170–82
Benabid A, Benazzous A, Pollak P (2002) Mechanisms of deep brain stimulation. Mov Disord 17:S73–S74
Benzi R, Sutera A, Vulpiani A (1999) The mechanism of stochastic resonance. J Phys A Math Gen 14:L453
Birdno M, Kuncel A, Dorval A, Turner D, Gross R, Grill W (2012) Stimulus features underlying reduced tremor suppression with temporally patterned deep brain stimulation. J Neurophysiol 107:364–83
Brette R, Guigon E (2003) Reliability of spike timing is a general property of spiking model neurons. Neural Comput 15:279–308
Bulsara A, Zador A (1996) Threshold detection of wideband signals: a noise-induced maximum in the mutual information. Phys Rev E Stat Phys Plasmas Fluids 54:R2185–R2188
Bulsara A, Jacobs E, Zhou T, Moss F, Kish L (1991) Stochastic resonance in a single neuron model: theory and analog simulation. J Theor Biol 152:531
Cecchi G, Sigman M, Alonso JM, Martinez L, Chialvo D, Magnasco M (2000) Noise in neurons is message dependent. Proc Natl Acad Sci USA 97:5557–61
Cook E, Johnston D (1997) Active dendrites reduce location-dependent variability of synaptic trains. J Neurophysiol 78:2116–28
Danziger Z, Grill W (2014) A neuron model of stochastic resonance using rectangular pulse trains. J Comput Neurosci 38:53–66
Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schfer H, Boetzel K, Danieks C, Deutschlnder A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Voges J (2006) A randomized trial of deep-brain stimulation for Parkinson’s disease. New Engl J Med 355:896–908
Durand DM, Kawaguchi M, Mino H (2013) Reverse stochastic resonance in a hippocampal ca1 neuron model. Conf Proc Ann Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Conf 2013:5242–5245
Faisal A, Selen L, Wolpert D (2008) Noise in the nervous system. Nat Rev Neurosci 9:292–303
Fauve S, Heslot F (1983) Stochastic resonance in a bistable system. Phys Lett A 97A:5–7
Gammaitoni L, Nggi P, Jung P, Marchesoni F (1999) Stochastic resonance. Rev Mod Phys 70
Grill W (2018) Temporal pattern of electrical stimulation is a new dimension of therapeutic innovation. Curr Opin Biomed Eng 8:1–6
Hänggi P (2002) Stochastic resonance in biology how noise can enhance detection of weak signals and help improve biological information processing. ChemPhysChem 3(3):285–290
Hänggi P, Jung P, Zerbe C, Moss F (1993) Can colored noise improve stochastic resonance? J Stat Phys 70:25–47
Holtzheimer P, Hamani C (2014) Deep brain stimulation for treatment-resistant depression, pp 64–75
Huang W, Zhang K (2019) Approximations of Shannon mutual information for discrete variables with applications to neural population coding. Entropy 21(3):243
Kawaguchi M, Mino H, Momose K, Durand DM (2009) Stochastic resonance can enhance information transmission of supra-threshold neural signals. Conf Proc Ann Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Conf 2009:6806–9
Kawaguchi M, Mino H, Durand DM (2011a) Stochastic resonance can enhance information transmission in neural networks. IEEE Trans Biomed Eng 58:1950–8
Kawaguchi M, Mino H, Momose K, Durand DM (2011b) Stochastic resonance with a mixture of sub-and supra-threshold stimuli in a population of neuron models. Conf Proc Ann Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Conf 2011:7328–31
Knoll G, Lindner B (2021) Recurrence-mediated suprathreshold stochastic resonance. J Comput Neurosci 49:407–418
Landa PS, McClintock P (2000) Vibrational resonance. J Phys A Math Gen 33:L433–L438
Lozano A, Lipsman N, Bergman H, Brown P, Chabardes S, Chang J, Matthews K, McIntyre C, Schlaepfer T, Schulder M, Temel Y, Volkmann J, Krauss J (2019) Deep brain stimulation: current challenges and future directions. Nat Rev Neurol 15:1
Mcdonnell M, Ward L (2011) The benefits of noise in neural systems: Bridging theory and experiment. Nat Rev Neurosci 12:415–425
Mina F (2013) Modeling the effects of direct and indirect electrical stimulation on neuronal dynamics: application to epilepsy (2013REN1S110). https://tel.archives-ouvertes.fr/tel-00955489
Mino H, Durand DM (2010) Enhancement of information transmission of sub-threshold signals applied to distal positions of dendritic trees in hippocampal ca1 neuron models with stochastic resonance. Biol Cybern 103:227–36
Moss F, Pierson D, O’Gorman D (1994) Stochastic resonance: tutorial and update. Int J Bifurcat Chaos 4:1375
Moss F, Ward L, Sannita W (2004) Stochastic resonance and sensory information processing: a tutorial and review of application. Clin Neurophysiol 115:267–81
Nozaki D, Mar D, Grigg P, Collins J (1999) Effects of colored noise on stochastic resonance in sensory neurons. Phys Rev Lett 82:2402–2405
Pycroft L, Stein J, Aziz T (2018) Deep brain stimulation: an overview of history, methods, and future developments. Brain Neurosci Adv 2:239821281881601
Riani M, Seife C, Roberts M, Twitty J, Moss F (1997) Visual perception of stochastic resonance. Phys Rev Lett 78:1186–1189
Santaniello S, Mccarthy M, Montgomery E, Gale J, Kopell N, Sarma S (2015) Therapeutic mechanisms of high-frequency stimulation in Parkinson’s disease and neural restoration via loop-based reinforcement
Snyder DL, Miller MI (1991) Random point processes in time and space, 2nd edn. Springer, New York
Stacey W, Durand D (2000) Stochastic resonance improves signal detection in hippocampal ca1 neurons. J Neurophysiol 83:1394–402
Stacey W, Durand D (2001) Synaptic noise improves detection of subthreshold signals in hippocampal ca1 neurons. J Neurophysiol 86:1104–12
Stacey W, Durand D (2002) Noise and coupling affect signal detection and bursting in a simulated physiological neural network. J Neurophysiol 88:2598–611
Stein R, Gossen E, Jones K (2005) Neuronal variability: noise or part of the signal? Nat Rev Neurosci 6:389–97
Stocks N, Mannella R (2001) Generic noise-enhanced coding in neuronal arrays. Phys Rev E Stat Nonlin Soft Matter Phys 64:030902
Vincent UE, McClintock PVE, Khovanov IA, Rajasekar S (2021) Vibrational and stochastic resonances in driven nonlinear systems. Philos Trans Roy Soc A Math Phys Eng Sci 379:20200226
Warman E, Durand D, Yuen G (1994) Reconstruction of hippocampal ca1 pyramidal cell electro-physiology by computer simulation. J Neurophysiol 71:2033–45
Yu Y, Wang X, Wang Q, Wang Q (2020) A review of computational modeling and deep brain stimulation: applications to Parkinson’s disease. Appl Math Mech 41:1747–1768
Zador A (1998) Impact of synaptic unreliability on the information transmitted by spiking neurons. J Neurophysiol 79:1219–29
Zhao J, deng B, Qin Y, Men C, Wang J, Wei X, Sun J (2017) Weak electric fields detectability in a noisy neural network. Cognit Neurodyn 11
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Benjamin Lindner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
1.1 Cable equation of pyramidal neuron model
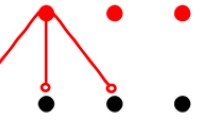
Fig. 1b shows the structure of a single pyramidal neuron model and the corresponding equivalent circuit whose cable equation is expressed as:
where \(V_m(t)\), \(C_m\), \(R_m\), \(R_a\), h and \(I_{\mathrm{syn}}(t)\) stand for membrane potential, membrane capacitance, membrane resistance, axial resistance, and infinitesimal length, and synaptic input from other neurons, respectively. The extracellular potential \(V_e(x,t)\) is represented as
where the \(\rho _{ext}\), and \(z_d\) denote extracellular resistivity, and the distance between the electrode and the fiber at x, respectively. The pulsatile HFS \(I_{\mathrm{pulse}}(t)\) is expressed as:
where \(I_{p}\) denotes the amplitude of pulsatile stimuli set at 14, 15, or 16 \(\mu A\), \(w=40\) \(\mu s\), and \(\tau _{\mathrm{int}}\) denotes the inverse of pulse frequency, and in which u(t) denotes a unit step function as follows:
The extracellular resistivity \(\rho _{ext}\) was set at 300.0 \(\Omega \cdot \)cm. In order to solve (A.1) with numerical calculations, the discretized version of (A.1) is obtained as follows:
where i, and k stand for the neuron number and the compartment number (\(i = 1,2,\dots ,N, k = 1,2,\dots ,26\)), \({V_m^{[i,k]}(t)}/{R_m^{[i,k]}} = 0\) at \(k=6\), \(I_{\mathrm{ion}}^{[i,k]}(t)=0\) at \(k\ne 6\), and \(I_{\mathrm{syn}}^{[i,k]}(t)\) denotes the synaptic stimuli coming from neurons other than ith neuron to the kth compartment determined randomly from 8th to 26th compartment in ith neuron (see Fig. 1b). To solve with Crank–Nicolson method, (A.5) is rearranged as follows:
1.2 Ion channel’s equation in soma
We assumed parallel equivalent circuit with seven conductances in the soma (Warman et al. 1994; Stacey and Durand 2000). Briefly, transmembrane current \(I^{[i,6]}_{\mathrm{ion}}(t)\) is described by
One sodium, three active potassium, one calcium, and two calcium-dependent potassium channel currents are incorporated into soma. These current can be expressed as follows:
where \(m_{[i]}(t)\), \(h_{[i]}(t)\) ,\(n_{[i]}(t)\), \(u_{[i]}(t)\), \(a_{[i]}(t)\), \(b_{[i]}(t)\), \(r_{[i]}(t)\), \(q_{[i]}(t)\), \(c_{[i]}(t)\) and \(d_{[i]}(t)\) are channel opening rates obtained by differential equations of each ion channel. These are determined by \(V_m^{[i,6]}(t)\), \(E_{\mathrm{rest}}\), \(g_x\) and \(E_x\), the membrane potential of soma, the resting potential of soma, conductance and reversal potential, respectively.
Rights and permissions
About this article
Cite this article
Mori, R., Mino, H. & Durand, D.M. Pulse-frequency-dependent resonance in a population of pyramidal neuron models. Biol Cybern 116, 363–375 (2022). https://doi.org/10.1007/s00422-022-00925-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-022-00925-w