Abstract
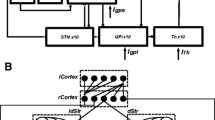
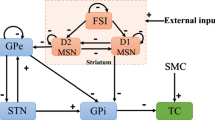
The cognitive impairment will gradually appear over time in Parkinson's patients, which is closely related to the basal ganglia-cortex network. This network contains two parallel circuits mediated by putamen and caudate nucleus, respectively. Based on the biophysical mean-field model, we construct a dynamic computational model of the parallel circuit in the basal ganglia-cortex network associated with Parkinson's disease dementia. The simulated results show that the decrease of power ratio in the prefrontal cortex is mainly caused by dopamine depletion in the caudate nucleus and is less related to that in the putamen, which indicates Parkinson's disease dementia may be caused by a lesion of the caudate nucleus rather than putamen. Furthermore, the underlying dynamic mechanism behind the decrease of power ratio is investigated by bifurcation analysis, which demonstrates that the decrease of power ratio is due to the change of brain discharge pattern from the limit cycle mode to the point attractor mode. More importantly, the spatiotemporal course of dopamine depletion in Parkinson's disease patients is well simulated, which states that with the loss of dopaminergic neurons projecting to the striatum, motor dysfunction of Parkinson's disease is first observed, whereas cognitive impairment occurs after a period of onset of motor dysfunction. These results are helpful to understand the pathogenesis of cognitive impairment and provide insights into the treatment of Parkinson's disease dementia.









Similar content being viewed by others
Data availability
We have read and have abided by the statement of ethical standards for manuscripts submitted to Neuroscience.
References
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9:357–381
Au WL, Zhou J, Palmes P, Sitoh Y, Tan LCS, Rajapakse JC (2012) Levodopa and the feedback process on set-shifting in Parkinson’s disease. Hum Brain Mapp 33:27–39
Blackwell KT, Czubayko KT, Plenz D (2003) Quantitative estimate of synaptic inputs to striatal neurons during up and down states in vitro. J Neurosci 23:9123–9132
Breakspear M (2017) Dynamic models of large-scale brain activity. Nat Neurosci 20:340–352
Calabresi P, Centonze D, Gubellini P, Marfia GA, Pisani A, Sancesario G, Bernardi G (2000) Synaptic transmission in the striatum: from plasticity to neurodegeneration. Prog Neurobiol 61:231–265
Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M (2014) Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 17(8):1022–1030
Carter DA, Fibiger HC (1977) Ascending projections of presumed dopamine-containing neurons in the ventral tegmentum of the rat as demonstrated by horseradish peroxidase. Neuroscience 2:569–576
Caviness JN, Hentz JG, Evidente VG, Driver-Dunckley E, Samanta J, Mahant P, Connor DJ, Sabbagh MN et al (2007) Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson’s disease. Parkinsonism Relat Disord 13:348–354
Christopher L, Marras C, Duff-Canning S, Koshimori Y, Chen R, Boileau I, Segura B, Monchi O et al (2004) Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson’s disease with mild cognitive impairment. Brain 137:565–575
Dirnberger G, Jahanshahi M (2013) Executive dysfunction in Parkinson’s disease: a review. J Neuropsychol 7:193–224
Frank MJ (2005) Dynamic dopamine modulation in the basal Ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci 17(1):51–72
Grafton ST (2004) Contributions of functional imaging to understanding Parkinsonian symptoms. Curr Opin Neurobiol 14:715–719
Grahn JA, Parkinson JA, Owen AM (2008) The cognitive functions of the caudate nucleus. Prog Neurobiol 86:141–155
Gratwicke J, Jahanshahi M, Foltynie T (2015) Parkinson’s disease dementia: a neural networks perspective. Brain 138:1454–1476
Hirano S (2021) Clinical implications for dopaminergic and functional neuroimage research in cognitive symptoms of Parkinson’s disease. Mol Med 27:40
Ilinsky IA, Tourtellotte WG, Kultas-Ilinsky K (1993) Anatomical distinctions between the two basal ganglia afferent territories in the primate motor thalamus. Stereotact Funct Neurosurg 60:62–69
Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Deerlin VV, Lee VMY, Leverenz JB et al (2012) Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 72:587–598
Leblois A, Boraud T, Meissner W, Bergman H, Hansel D (2006) Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia. J Neurosci 26(13):3567–3583
Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM (2003) Cognitive impairments in Early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci 23(15):6351–6356
Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, Hyde TM, Weinberger DR (2002) Dopaminergic Modulation of cortical function in patients with Parkinson’s disease. Ann Neurol 51:156–164
Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A (2004) Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci 24(3):702–710
Monchi O, Petrides M, Mejia-Constain B, Strafella AP (2007) Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain 130:233–244
Morita A, Kamei S, Mizutani T (2011) Relationship between slowing of the EEG and cognitive impairment in Parkinson disease. J Clin Neurophysiol 28:384–387
Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9:856–869
Nakano K (2000) Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev 22:S5–S16
Neufeld MY, Blumen S, Aitkin I, Parmet Y, Korczyn AD (1994) EEG frequency analysis in demented and nondemented Parkinsonian patients. Dementia 5:23–28
Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW (2000) Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci 23(Suppl.):S8–S19
Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RSJ, Brooks DJ (1992) Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol 32:151–161
Pugnetti L, Baglio F, Farina E, Alberoni M, Calabrese E, Gambini A, Bella ED, Garegnani M et al (2010) EEG evidence of posterior cortical disconnection in PD and related dementias. Int J Neurosci 120:88–98
Rennie CJ, Robinson PA, Wright JJ (1999) Effects of local feedback on dispersion of electrical waves in the cerebral cortex. Phys Rev E 59:3320–3329
Rinne JO, Rummukainen J, Paljiarvi L, Rinne UK (1989) Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Ann Neurol 26:47–50
Roberts JA, Robinson PA (2008) Modeling absence seizure dynamics: implications for basic mechanisms and measurement of thalamocortical and corticothalamic latencies. J Theor Biol 253:189–201
Robinson PA, Rennie CJ, Rowe DL, O’Connor SC (2004) Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Hum Brain Mapp 23:53–72
Rubin JE, Terman D (2004) High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J Comput Neurosci 16:211–235
Sieveritz B, Arbuthnott GW (2020) Prelimbic cortical targets of ventromedial thalamic projections include inhibitory interneurons and corticostriatal pyramidal neurons in the rat. Brain Struct Funct 225:2057–2076
Soikkeli R, Partanen J, Soininen H, Pääkkönen A, Riekkinen P (1991) Slowing of EEG in Parkinson’s disease. Clin Neurophysiol 79:159–165
Stoffers D, Bosboom JLW, Deijen JB, Wolters EC, Berendse HW, Stam CJ (2007) Slowing of oscillatory brain activity is a stable characteristic of Parkinson’s disease without dementia. Brain 130:1847–1860
Taylor JP, O’Brien JT (2012) Parkinson’s disease with dementia. Adv Biol Psych 27:103–124
Terman D, Rubin JE, Yew AC, Wilson CJ (2002) Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J Neurosci 22:296–2976
Van Albada SJ, Robinson PA (2009) Mean-field modeling of the basal ganglia-thalamocortical system. I Firing rates in healthy and Parkinsonian states. J Theor Biol 257:642–663
Van Albada SJ, Gray RT, Drysdale PM, Robinson PA (2009) Mean-field modeling of the basal ganglia-thalamocortical system. II. Dynamics of Parkinsonian oscillations. J Theor Biol 257:664–688
Wichmann T, DeLong MR (1996) Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol 6:751–758
Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR (1999) Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia Nigra Pars reticulata in primates. Exp Brain Res 125:397–409
Yu Y, Zhang HH, Zhang LY, Wang QY (2019) Dynamical role of pedunculopntine nucleus stimulation on controlling Parkinson’s disease. Physica A 525:834–848
Yu Y, Wang XM, Wang QS, Wang QY (2020) A review of computational modeling and deep brain stimulation: applications to Parkinson’s disease. Appl Math Mech-Engl 41(12):1747–1768
Zimmermann R, Gschwandtner U, Hatz F, Schindler C, Bousleiman H, Ahmed S, Hardmeier M, Meyer A et al (2015) Correlation of EEG slowing with cognitive domains in nondemented patients with Parkinson’s Disease. Dement Geriatr Cogn Disord 39:207–214
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (Grant Nos. 12372062, 11972217), the excellent Graduate Training Program of Shaanxi Normal University (LHRCTS23056).
Author information
Authors and Affiliations
Contributions
Hao Yang and XiaoLi Yang contributed to the conception of this work and wrote the main manuscript text. SiLu Yan performed the analysis and proposed constructive advice. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no potential conflict of interest.
Additional information
Communicated by Benjamin Lindner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Yang, X. & Yan, S. A dynamic computational model of the parallel circuit on the basal ganglia-cortex associated with Parkinson’s disease dementia. Biol Cybern 118, 127–143 (2024). https://doi.org/10.1007/s00422-024-00988-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-024-00988-x