Abstract
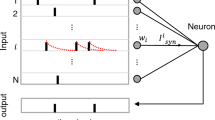
Tritonia has been studied in the laboratory by several studies, which have led to significant advances in identifying the biological components that participate in the Tritonia escape swim network. There are also studies, which have artificially reproduced the neuronal patterns of the Tritonia escape swim circuit. These studies simulated the interneurons of the swim central pattern generator (CPG) known as dorsal swim interneuron, ventral swim interneuron, and cerebral 2. In this research, other neurons that participate in the Tritonia escape swim network were simulated. In addition to the main CPG components, sensory, ramp, and dorsal/ventral flexion neurons are all included in the neural network (NN). The objective of the study was to artificially reconstruct a more representative image of the Tritonia escape swim NN, its neuronal activities, and synaptic connections. The network was simulated using a spiking neural network (SNN) simulator named Synapse, which has been implemented based on a novel event-based SNN algorithm. After tuning synaptic delays, weights, and membrane potential properties, the expected spike patterns were successfully reproduced for each involved neuron. The spike patterns from this study were validated using the laboratory recorded signals as well as the existing simulated patterns.










Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
All data generated or analyzed during this study are included in this published article.
References
Lee AH, Megalou EV, Wang J, Frost WN (2012) Axonal conduction block as a novel mechanism of prepulse inhibition. J Neurosci 32(44):15262–15270
Getting PA (1983) Mechanisms of pattern generation underlying swimming in Tritonia. III. Intrinsic and synaptic mechanisms for delayed excitation. J Neurophysiol 49(4):1036–1050
Katz PS, Sakurai A, Clemens S, Davis D (2004) Cycle period of a network oscillator is independent of membrane potential and spiking activity in individual central pattern generator neurons. J Neurophysiol 92(3):1904–1917
Frost WN, Hoppe TA, Wang J, Tian LM (2001) Swim initiation neurons in Tritonia diomedea. Am zool 41(4):952–961
Getting PA (1976) Afferent neurons mediating escape swimming of the marine mollusc, Tritonia. J Comp Physiol 110(3):271–286
Getting PA (1981) Mechanisms of pattern generation underlying swimming in Tritonia. I. Neuronal network formed by monosynaptic connections. J Neurophysiol 46(1):65–79
Getting PA (1983) Mechanisms of pattern generation underlying swimming in Tritonia. II. Network reconstruction. J Neurophysiology 49(4):1017–1035
Hume RI, Getting PA (1982) Motor organization of Tritonia swimming. II. Synaptic drive to flexion neurons from premotor interneurons. J Neurophysiol 47(1):75–90
Katz PS, Frost WN (1995) Intrinsic neuromodulation in the Tritonia swim CPG: the serotonergic dorsal swim interneurons act presynaptically to enhance transmitter release from interneuron C2. J Neurosci 15(9):6035–6045
Katz PS (2009) Tritonia swim network. Scholarpedia 4(5):3638
Mongeluzi DL, Frost WN (2000) Dishabituation of the Tritonia escape swim. Learn Mem 7(1):43–47
Wyeth R, Woodward O (2012) Tritonia diomedea escape swim behaviour. YouTube. YouTube https://www.youtube.com/watch?v=JCr1b1mJWoU
Sakurai A, Katz PS (2009) Functional recovery after lesion of a central pattern generator. J Neurosci 29(42):13115–13125
Balaban PM (1979) A system of command neurons in snail’s escape behavior. Acta Neurobiol Exp 39:97–107
Calin-Jageman RJ, Tunstall MJ, Mensh BD, Katz PS, Frost WN (2007) Parameter space analysis suggests multi-site plasticity contributes to motor pattern initiation in Tritonia. J Neurophysiol 98(4):2382–2398
Bichler O, Querlioz D, Thorpe SJ, Bourgoin J-P, Gamrat C (2012) Extraction of temporally correlated features from dynamic vision sensors with spike-timing-dependent plasticity. Neural Netw 32:339–348
Gerstner W (2001) A framework for spiking neuron models: the spike response model. In: Handbook of biological physics, vol. 4. North-Holland, pp 469–516
Carlson KD, Nageswaran JM, Dutt N, Krichmar JL (2014) An efficient automated parameter tuning framework for spiking neural networks. Front neurosci 8:10
Fickbohm DJ, Katz PS (2000) Paradoxical actions of the serotonin precursor 5-hydroxytryptophan on the activity of identified serotonergic neurons in a simple motor circuit. J Neurosci 20(4):1622–1634
Frost WN, Tian L-M, Hoppe TA, Mongeluzi DL, Wang J (2003) A cellular mechanism for prepulse inhibition. Neuron 40(5):991–1001
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miri, F., Miles, C.I. & Lewis, H.W. Simulating a complete Tritonia escape swim network using a novel event-based spiking neural network algorithm. Neural Comput & Applic 35, 1733–1748 (2023). https://doi.org/10.1007/s00521-022-07829-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-022-07829-7