Abstract
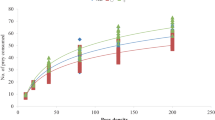
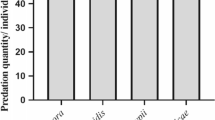
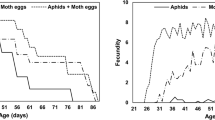
Protein and carbohydrate content in the diet of the predator Hippodamia variegata Goeze (variegated ladybug) directly influences reproduction fitness by affecting foraging efficiency. The effect of three varied qualities of Aphis gossypii Glover (cotton aphid) prey on daily H. variegata feeding and daily egg production (DEP) were estimated using the support vector machine (SVM). The SVM can predict predator reproductive behavior as a function of the relationship between foraging and prey nutritional composition. We used the total number and weight of aphids consumed, volume of protein, lipid, carbohydrate, and glycogen, and total energy received by the ladybug after feeding on one prey item as input variables. Aphid quality varied as nitrogen (N) fertilization levels were 110, 160, and 210 ppm on the Cucumis sativus L. (cucumber) host plants. The model estimated female beetles consumed more aphids and nutrients on C. sativus plants with N levels of 160 ppm compared to lower (110 ppm) N levels and had higher reproductive transformation efficiencies. Transformation rates of aphid feeding to egg production in females exposed to the 160 ppm treatment were 65% greater, had lower nutrient and energy requirements, and achieved a 29.4% higher DEP than those exposed to high (210 ppm) N levels. The SVM predicted nutrient compositions of A. gossypii exposed to 160 ppm N were balanced such that H. variegata exhibited greater reproduction efficiency than the other N treatments for first 30 d from the start of reproduction.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ANN:
-
Artificial neural network
- Avg:
-
Average
- Carb:
-
Carbohydrate
- Const:
-
Constant
- DEP:
-
Daily egg production, \(\hat{y}\)
- GA:
-
Genetic algorithm
- Gly:
-
Glycogen
- Lip:
-
Lipid
- MAPE:
-
Mean absolute percent error
- N:
-
Nitrogen
- P:
-
Protein
- Poly:
-
Polynomial
- Q p :
-
Number aphids consumed/eggs produced
- R 2 :
-
Coefficient of determination
- R:
-
Reproduction
- RBF:
-
Radial basis function
- RMSE:
-
Root mean squared error
- TC:
-
Total daily aphid consumption
- TE:
-
Total energy
- SVM:
-
Support vector machine
- V :
-
Variance
- TCw:
-
Consumed aphid weight
References
Obrycki JJ, Orr CJ (1990) Suitability of three prey species for nearctic populations of Coccinella septempunctata, Hippodamia variegata, and Propylea quatuordecimpunctata (Coleoptera: Coccinellidae). J Econ Entomol 83:1292–1297
Michaud JP (2000) Development and reproduction of ladybeetles (Coleoptera: Coccinellidae) on the citrus aphids Aphis spiraecola Patch and Toxoptera citricida (Kirkaldy) (Homoptera: Aphididae). Biol Control 18:287–297
Schmidt JM et al (2012) The nutritional content of prey affects the foraging of a generalist arthropod predator. PLoS ONE 7:e49223
Jensen K et al (2012) Optimal foraging for specific nutrients in predatory beetles. Proc R Soc B 279:2212–2218
Hosseini A et al (2019) Life history responses of Hippodamia variegata (Coleoptera: Coccinellidae) to changes in the nutritional content of its prey, Aphis gossypii (Hemiptera: Aphididae), mediated by nitrogen fertilization. Biol Control 130:27–33
Dmitriew C, Rowe L (2011) The effects of larval nutrition on reproductive performance in a food-limited adult environment. PLoS ONE 6(3):e17399
Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW et al (2008) Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci USA 105:2498–2503
Carey J et al (2003) Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell 1:140–148
Jensen K et al (2015) Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell 14:605–615
Tu M-P, Tatar M (2003) Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell 2(6):327–333
Kouloussis NA et al (2017) Age related assessment of sugar and protein intake of Ceratitis capitata in ad libitum conditions and modeling its relation to reproduction. Front Physiol 8:271
Hunt J et al (2004) High-quality male field crickets invest heavily in sexual display but die young. Lett Nat 432:1024–1027
Maklakov AA et al (2008) Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr Biol 18:1062–1066
Kessler A (1971) Relation between egg production and food consumption in species of the genus Pardosa (Lycosidae, Araneae) under experimental conditions of food-abundance and food-shortage. Oecologia 8(1):93–109
Toft S, Li D, Mayntz D (2010) A specialized araneophagic predator’s short-term nutrient utilization depends on the macronutrient content of prey rather than on prey taxonomic affiliation. Physiol Entomol 35(4):317–327
Wilder SM, Rypstra AL (2008) Diet quality affects mating behaviour and egg production in a wolf spider. Anim Behav 76:438–444
Jensen K et al (2011) Nutrient regulation in a predator, the wolf spider Pardosa prativaga. Anim Behav 81:993–999
Chen Y, Olson DM, Ruberson JR (2010) Effects of nitrogen fertilization on tritrophic interactions. Arthropod Plant Interact 4:81–94
Aqueel MA, Leather SR (2011) Effect of nitrogen fertilizer on the growth and survival of Rhopalosiphum padi (L.) and Sitobion avenae (F.) (Homoptera: Aphididae) on different wheat cultivars. Crop Prot 30(2):216–221
Aqueel MA, Leather SR (2012) Nitrogen fertiliser affects the functional response and prey consumption of Harmonia axyridis (Coleoptera: Coccinellidae) feeding on cereal aphids. Ann Appl Biol 160:6–15
Couture JJ, Servi JS, Lindroth RL (2010) Increased nitrogen availability influences predator–prey interactions by altering host-plant quality. Chemoecology 20:277–284
Han P et al (2015) Effect of plant nitrogen and water status on the foraging behavior and fitness of an omnivorous arthropod. Ecol Evol 5:5468–5477
Hosseini A et al (2018) Nitrogen fertilization increases the nutritional quality of Aphis gossypiim (Hemiptera: Aphididae) as prey for Hippodamia variegata (Coleoptera: Coccinellidae) and alters predator foraging behavior. J Econ Entomol 111:2059–2068
Michaud JP (2005) On the assessment of prey suitability in aphidophagous Coccinellidae. Eur J Entomol 102:385–390
Aqueel MA et al (2013) Effect of plant nutrition on aphid size, prey consumption, and life history characteristics of green lacewing. Insect Sci 21:74–82
De Clercq P, Mohaghegh J, Tirry L (2000) Effect of host plant on the functional response of the predator Podisus nigrispinus (Heteroptera: Pentatomidae). Biol Control 18:65–70
Wu ZH, Zhou ZR, Pang BP (2010) Influence of five host plants of Aphis gossypii Glover on some population parameters of Hippodamia variegata (Goeze). J Pest Sci 83:77–83
Chen Y (2008) Support vector machines and fuzzy systems. In: Maimon O, Rokach L (eds) Soft computing for knowledge discovery and data mining. Springer, Boston, pp 205–223
Zadeh LA (1993) Fuzzy logic, neural networks and soft computing. In: Natke HG, Tomlinson GR, Yao JTP (eds) Safety evaluation based on identification approaches related to time-variant and nonlinear structures. Vieweg+Teubner Verlag, Wiesbaden, pp 320–321
Rohani A, Mamarabadi M (2018) Free alignment classification of dikarya fungi using some machine learning methods. Neural Comput Appl 1–22
Vapnik V (1995) The nature of statistical learning theory. Springer-Verlag, New York
Sadeghi R et al (2012) Use of support vector machines (SVMs) to predict distribution of an invasive water fern Azolla filiculoides (Lam.) in Anzali wetland, southern Caspian Sea, Iran. Ecol Model 244:117–126
Honěk A (1985) Habitat preferences of aphidophagous coccinellids [Coleoptera]. Entomophaga 30:253–264
Farhadi R, Allahyari H, Juliano SA (2010) Functional response of larval and adult stages of Hippodamia variegata (Coleoptera: Coccinellidae) to different densities of Aphis fabae (Hemiptera: Aphididae). Environ Entomol 39:1586–1592
Madadi H et al (2011) Assessment of the biological control capability of Hippodamia varigata (Col: Coccinellidae) using functional response experiments. J Pest Sci 84:447–455
Skouras PJ et al (2015) Development, growth, feeding and reproduction of Ceratomegilla undecimnotata, Hippodamia variegata and Coccinella septempunctata fed on the tobacco aphid, Myzus persicae nicotianae. Phytoparasitica 43:159–169
Holland JH (1975) Adaption in natural and artificial system. The MIT Press, Massachusetts
Raikar RV et al (2016) Prediction of contraction scour using ANN and GA. Flow Meas Instrum 50:26–34
Gholipoor M, Rohani A, Torani S (2013) Optimization of traits to increasing barley grain yield using an artificial neural network. Int J Plant Prod 7:1–18
Esmaeilpour-Troujeni M, Rohani A, Khojastehpour M (2021) Optimization of rapeseed production using exergy analysis methodology. Sustain Energy Technol Assess 43:100959
Rahimi M, Abbaspour-Fard MH, Rohani A (2021) Machine learning approaches to rediscovery and optimization of hydrogen storage on porous bio-derived carbon. J Clean Prod 329:129714
Rahimi M, Pourramezan M-R, Rohani A (2022) Modeling and classifying the in-operando effects of wear and metal contaminations of lubricating oil on diesel engine: a machine learning approach. Expert Syst Appl 203:117494
McCall J (2005) Genetic algorithms for modelling and optimisation. J Comput Appl Math 184:205–222
Inc SI (2004) SAS/STAT user’s guide, version 9.1, vols 1 and 2. SAS Institute Inc., Cary
Simpson SJ, Raubenheimer D (2005) Obesity: the protein leverage hypothesis. Obes Rev 6(2):133–142
Martinez-Cordero C et al (2012) Testing the protein leverage hypothesis in a free-living human population. Appetite 59:312–315
Jensen K et al (2013) Balancing of specific nutrients and subsequent growth and body composition in the slug Arion lusitanicus. Physiol Behav 122:84–92
Ruohonen K, Simpson S, Raubenheimer D (2007) A new approach to diet optimisation: a re-analysis using European whitefish (Coregonus lavaretus). Aquaculture 267:147–156
Raubenheimer D, Simpson SJ, Mayntz D (2009) Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct Ecol 23(1):4–16
Brown HD (1974) Defensive behaviour of the wheat aphid, Schizaphis graminum (Rondani) (Hemiptera: Aphididae), against Coccinellidae. J Entomol Ser A Gen Entomol 48(2):157–165
Wu G-M et al (2010) Altruistic defence behaviours in aphids. BMC Evol Biol 10(1):19
Glendinning JI (2007) How do predators cope with chemically defended foods? Biol Bull 213(3):252–266
Rafter JL et al (2017) Impact of consuming ‘toxic’ monarch caterpillars on adult Chinese mantid mass gain and fecundity. Insects 8(1):23
Skelhorn J, Rowe C (2007) Predators’ toxin burdens influence their strategic decisions to eat toxic prey. Curr Biol 17(17):1479–1483
Barrett ELB et al (2009) Separate and combined effects of nutrition during juvenile and sexual development on female life-history trajectories: the thrifty phenotype in a cockroach. Proc R Soc B Biol Sci 276(1671):3257–3264
Plesnar-Bielak A et al (2017) Larval and adult nutrition effects on reproductive traits in the red flour beetle. J Zool 302(2):79–87
Aguila JR, Hoshizaki DK, Gibbs AG (2012) Contribution of larval nutrition to adult reproduction in Drosophila melanogaster. J Exp Biol 216:399–406
Bressendorff B, Toft S (2011) Dome-shaped functional response induced by nutrient imbalance of prey. Biol Lett 7:517–520
Hunt J, Brooks R, Jennions MD (2005) Female mate choice as a condition-dependent life-history trait. Am Nat 166:79–92
Mueller P, Diamond J (2001) Metabolic rate and environmental productivity: well-provisioned animals evolved to run and idle fast. Proc Natl Acad Sci USA 98:12550–12554
Forsman A (1996) Body size and net energy gain in gape-limited predators: a model. J Herpetol 30:307–319
Koemel NA, Barnes CL, Wilder SM (2019) Metabolic and behavioral responses of predators to prey nutrient content. J Insect Physiol 116:25–31
Jensen K et al (2010) Metabolic consequences of feeding and fasting on nutritionally different diets in the wolf spider Pardosa prativaga. J Insect Physiol 56(9):1095–1100
Hall DG (2001) Notes on the yellow sugarcane aphid Sipha flava (Homoptera: Aphididae) and the lady beetle Diomus terminatus (Coleoptera: Coccinellidae) in Florida. J Am Soc Sugar Cane Technol 21:21–29
van Lenteren JC (2003) Need for quality control of mass-produced biological control agents. In: van Lenteren JC (ed) Quality control and production of biological control agents: theory and testing procedures. CABI Publishing, Wallingford, pp 1–18
Simpson SJ, Raubenheimer D (1995) The geometric analysis of feeding and nutrition: a user’s guide. J Insect Physiol 41(7):545–553
Mayntz D et al (2009) Balancing of protein and lipid intake by a mammalian carnivore, the mink, Mustela vison. Anim Behav 77(2):349–355
Raubenheimer D et al (2007) Nutrient-specific compensation following diapause in a predator: implications for intraguild predation. Ecology 88:2598–2608
Acknowledgements
The authors are grateful to the Ferdowsi University of Mashhad for Grant No. P3/33503 which provided financial support for this research. The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseini, A., Hosseini, M., Rohani, A. et al. Modeling reproductive fitness of predator, Hippodamia variegata (Coleoptera: Coccinellidae) using support vector machine (SVM) on three nitrogen treatments. Neural Comput & Applic 35, 24333–24346 (2023). https://doi.org/10.1007/s00521-023-09020-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-023-09020-y