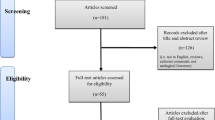
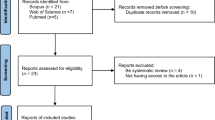
Abstract
Deep learning integration in cancer diagnosis enhances accuracy and diagnosis speed which helps clinical decision-making and improves health outcomes. Despite all these benefits in cancer diagnosis, the present AI models in urology cancer diagnosis have not been sufficiently reviewed systematically. This paper reviews the artificial intelligence approaches used in cancer diagnosis, prediction, and treatment of urology cancer. AI models and their applications in urology subspecialties are evaluated and discussed. The Scopus, Microsoft Academic and PubMed/MEDLINE databases were searched in November 2022 using the terms “artificial intelligence”, “neural network”, “machine learning,” or “deep learning” combined with the phrase “urology cancers”. The search was limited to publications published within the previous 20 years to identify cutting-edge deep-learning applications published in English. Irrelevant review articles and publications were eliminated. The included research involves two kinds of research analysis: quantitative and qualitative. 48 articles were included in this survey. 25 studies proposed several approaches for prostate cancers, while 15 were for bladder cancers. 8 studies discussed renal cell carcinoma and kidney cancer. The models presented to detect urology cancers have achieved high detection accuracy (77–95%). Deep learning approaches that use convolutional neural networks have achieved the highest accuracy among other techniques. Although it is still progressing, the development of AI models for urology cancer detection, prediction, and therapy has shown significant promise. Additional research is required to employ more extensive, higher-quality, and more recent datasets to the clinical performance of the proposed AI models in urology cancer applications.








Similar content being viewed by others
Data availability
Data is available on request from the authors.
References
NIH (2021) Home American cancer society–cancer facts statistics. [Online] Available: https://cancerstatisticscenter.cancer.org/?_ga=2.191090925.69577. 2 Jan 2837.1589031645-278983135.1589031645#!/
Fass L (2008) Imaging and cancer: a review. Mol Oncol 2:115–152
Doi K (2007) Computer-aided diagnosis in medical imaging: historical review, current status and future potential. Comput Med Imaging Gr 31:198–211
Suarez-Ibarrola R, Basulto-Martinez M, Heinze A, Gratzke C, Miernik A (2018) Radiomics applications in renal tumor assessment: a comprehensive review of the literature. Cancers (Basel) 12(6):3–17
Erak E, Oliveira LD, Mendes AA, Dairo O, Ertunc O, Kulac I, Baena-Del Valle JA, Jones T, Hicks JL, Glavaris S, Guner G, Vidal ID, Markowski M, d. l. Calle (2023) Predicting prostate cancer molecular subtype with deep learning on histopathologic images. Mod Pathol 36(10):0893–3952
Hamm CA, Baumgärtner GL, Biessmann F, Beetz NL, Hartenstein A, Savic LJ, Froböse K, Dräger F, Schallenberg S, Rudolph M, Baur ADJ, Hamm B, Haas M, Hofbauer S (2023) Interactive explainable deep learning model informs prostate cancer diagnosis at MRI. Radiol Soc North Am 307(4):e222276
N. W. T. C. M. L. B. Xu (2015) Empirical evaluation of rectified activations in convolutional network. arXiv preprint. 1505.00853
Pacal I, Karaboga D, Basturk A, Akay B, Nalbantoglu U (2020) A comprehensive review of deep learning in colon cancer. Comput Biol Med 126(104003):0010–4825
Kim J-E, Nam N-E, Shim J-S, Jung Y-H, Cho B-H, Hwang J (2020) Transfer learning via deep neural networks for implant fixture system classification using periapical radiographs. J Clin Med 9(4):1117
Yulian Z, Wang L, Mingxia L, Chunjun Q, Ambereen Y, Aytekin O, Dinggang S (2017) MRI-based prostate cancer detection with high-level representation and hierarchical classification. Am Assoc Phys Med 44(3):1028–1039
Baghdadi A, Aldhaam NA, Elsayed AS, Hussein AA, Cavuoto LA, Kauffman E, Guru KA (2020) Automated differentiation of benign renal oncocytoma and chromophobe renal cell carcinoma on computed tomography using deep learning. BJU int 125(4):553–560
Tanaka T, Huang Y, Marukawa Y, Tsuboi Y, Masaoka Y, Kojima K, Iguchi T, Hiraki T, Gobara H, Yanai H, Nasu Y, Kanazawa S (2020) Differentiation of small (≤ 4 cm) renal masses on multiphase contrast-enhanced CT by deep learning. Am J Roentgenol 214(3):3–11
Leyh-Bannurah S-R, Tian Z, Karakiewicz PI, Wolffgang U, Sauter G, Fisch M, Pehrke D, Huland H, Graefen M, Budäus L (2018) Deep learning for natural language processing in urology: state-of-the-art automated extraction of detailed pathologic prostate cancer data from narratively written electronic health records. JCO Clin Cancer Inform 2:1–9
Wang X, Guo J, Gu D, Yang Y, Yang X, Zhu K (2019) Tracking knowledge evolution, hotspots and future directions of emerging technologies in cancers research: a bibliometrics review. J Cancer 10(12):2643
Singhal N, Soni S, Bonthu S, Chattopadhyay N, Samanta P, Joshi U, Jojera A, Chharchhodawala T, Agarwal A, Desai M, Ganpule A (2022) A deep learning system for prostate cancer diagnosis and grading in whole slide images of core needle biopsies. Sci Rep 12:3383
Bulten W, Litjens G, Pinckaers H, Ström P, Eklund M, Kartasalo K Demkin M, Dane S (2020) The PANDA challenge: prostate cANcer graDe assessment using the gleason grading system. In: 23rd international conference on medical image computing and computer assisted intervention (MICCAI 2020)
Lin F, Ma C, Xu J, Lei Y, Li Q, Lan Y, Sun M, Long W, Cui E (2020) A CT-based deep learning model for predicting the nuclear grade of clear cell renal cell carcinoma. Eur J Radiol 129:1–15
Park JS, Lee HJ, Cho NH, Kim J, Jang WS, Heo JE, Ham WS (2019) "Risk prediction tool for aggressive tumors in clinical T1 stage clear cell renal cell carcinoma using molecular biomarkers. Comput Struct Biotechnol J 17:371–377
Okyaz E, Nurettin E, Axel S, Bernhard B (2018) Diagnostic classification of cystoscopic images using deep convolutional neural networks. JCO Clin Cancer Inform 2:1–8
Atsushi I, Hirokazu N, Kochi, Hiroyuki N, Hidenori S (2018) Objective evaluation for the cystoscopic diagnosis of bladder cancer using artificial intelligence. European Urology Supplements. e1230–e1231
Shkolyar E, Jia X, Xing L, Liao J (2019) Automated cystoscopic detection of bladder cancer using deep-Learning. J Urol. https://doi.org/10.1097/01.JU.0000557512.76700.42
Atsushi I, Hirokazu N, Yuta K, Hiromitsu N, Takahiro K, Hidenori S, Masahiro M, Hiroyuki N (2020) Cystoscopic imaging for bladder cancer detection based on stepwise organic transfer learning with a pretrained convolutional neural network. J endourol. https://doi.org/10.1089/end.2020.0919
Eric W, Lubomir M, Ravi K, Heang-Ping C, Kenny H, Caleb R, Richard H, Elaine M, Chintana P, Ajjai A, Alon Z (2019) Deep Learning approach for assessment of bladder cancer treatment response,". Tomography 5(201):8. https://doi.org/10.18383/j.tom.2018.00036
Kenny H, Lubomir M, Richard H, Heang-Ping C, Elaine M, Matthew S, Ravi K, Alon Z, Ajjai A, Galina K-N, Kimberly S, Nathaniel M, Daniel B, Sean W, Prasad R, Isaac R (2019) Diagnostic accuracy of CT for prediction of bladder cancer treatment response with and without computerized decision support. Acad Radiol 26(9):1137–1145. https://doi.org/10.1016/j.acra.2018.10.010
Takumi T, Mami H-K, Yumiko O, Satoshi I, Koji M (2019) Prediction of prostate cancer by deep learning with multilayer artificial neural network. Can Urol Assoc 13(5):E145–E150. https://doi.org/10.5489/cuaj.5526
Andrew J, Jian C, Saum G, Paul J, Zequn L, Jessica N, Sanjay P, Inderbir S, Yan L (2019) A deep-learning model using automated performance metrics and clinical features to predict urinary continence recovery after robot-assisted radical prostatectomy. BJU Int 124(3):487–495. https://doi.org/10.1111/bju.14735
Junichiro I, Yoh M, Sho U, Yosuke Y, Toshiki K, Soichiro Y, Minato Y, Kazutaka S, Kazunori K, Noboru N, Tomo K, Kosei K, Itsuo K, Yasuhisa F (2018) “Computer-aided diagnosis of prostate cancer on magnetic resonance imaging using a convolutional neural network algorithm.” BJU int 122(3):411–417
Ma L, Guo R, Zhang G, Tade F, Schuster DM, Nieh P, Master V, Fei B (2017) Automatic segmentation of the prostate on CT images using deep learning and multi-atlas fusion. Med Imaging: Image Processing 10133:760–768
Oberai A, Varghese B, Cen S, Angelini T, Hwang D, Gill I, Aron M, Lau C, Duddalwar V (2020) "Deep learning based classification of solid lipid-poor contrast enhancing renal masses using contrast enhanced CT. The Br J Radiol 93:11–31
Cha KH, Hadjiiski L, Chan H-P, Weizer AZ, Alva A, Cohan RH, Caoili EM, Paramagul C, Samala RK (2017) Bladder cancer treatment response assessment in CT using radiomics with deep-learning. Sci Rep 7(1):8738
Lucas M, Liem EI, Savci-Heijink CD, Freund JE, Marquering HA, van Leeuwen TG, de Bruin DM (2019) Toward automated in vivo bladder tumor stratification using confocal laser. J Endurol 33(11):930–937
Cha KH, Hadjiiski LM, Samala RK, Chan H-P, Cohan RH, Caoili EM, Paramagul C, Alva A, Weizer AZ (2016) Bladder cancer segmentation in CT for treatment response assessment: application of deep-learning convolution neural network: a pilot study. Tomography 2(4):421–429
Harmon SA, Sanford TH, Brown T, Yang C, Mehralivand S, Jacob JM, Valera VA, Shih JH, Agarwal PK, Choyke PL, Turkbey B (2020) Multiresolution application of artificial intelligence in digital pathology for prediction of positive lymph nodes from primary tumors in bladder cancer. Orig Rep 4:367–382
Hadjiiski LM, Cha KH, Cohan RH, Chan H-P, Caoili EM, Davenport MS, Samala RK, Weizer AZ, Alva A, Kirova-Nedyalkova G, Shampain K, Meyer N, Barkmeier D, Woolen SA, Shankar PR (2020) Intraobserver variability in bladder cancer treatment response assessment with and without computerized decision support. TomoGraphy 6(2):194–202
Ilaria J, Marit L, Judith B, Onno J, Sybren L, Ton G, Henk A, Jakko A, Daniel M (2020) Automated detection and grading of non-muscle-invasive urothelial cell carcinoma. Am J Pathol 190(7):1483–1490
Wu E, Hadjiiski LM, Samala RK, Chan HP, Cha KH, Richter C, Cohan RH, Caoili EM, Paramagul C, Alva A, Weizer AZ (2019) Deep learning approach for assessment of bladder cancer treatment response. TomoGraphy 5(1):201–208
Cha KH, Hadjiiski LM, Cohan RH, Chan HP, Caoili EM, Davenport MS, Samala RK, Weizer AZ, Alva A, Kirova-Nedyalkova G, Shampain K (2019) Diagnostic accuracy of CT for prediction of bladder cancer treatment response with and without computerized decision support. Acad Radiol 26(9):1137–1145
Atsushi I, Hirokazu N, Yuta K, Takahiro K, Koji K, Hidenori S, Masahiro M, Hiroyuki N (2020) Support system of cystoscopic diagnosis for bladder cancer based on artificial. J Endurol 34(3):352–358
Alexander U, Michelle B, Justin G-B, Edward U, Chanon C, Michael N, Daniel C, Peter D, Roozbeh H (2020) A 3D/2D Hybrid U-Net CNN approach to prostate organ segmentation of mpMRI. Am J Roentgenol 216(1):111–116
dBJ and WA (2020) “Deep learning in MIBC” Research Hihglights
Eirini A, Kim S, Michael M, Niels R, Thomas H, Christian F, Norbert W, Peter J, Jan H, Manfred C (2018) Automated gleason grading of prostate cancer tissue microarrays via deep learning. Sci Rep 8(1):1–11
Ke Y, Xiuying W, Jinman K, Mohamed K, Michael F, Dagan F (2019) A propagation-DNN: deep combination learning of multi-level features for MR prostate segmentation. Comput Methods Progr Biomed 170:11–21
Patrick S, Simon K, Jan P, Manuel W, Philipp K, Sebastian B, Tristan A, Albrecht S, Markus H, Heinz-Peter S, Klaus H-H, David B (2019) Classification of cancer at prostate MRI: deep learning versus clinical PI-RADS assessment. Radiology 293(3):607–617
Reda I, Shalaby A, Elmogy M, Elfotouh AA, Khalifa F, El-Ghar MA, Hosseini-Asl E, Gimel’farb G, Werghi N, El-Baz A (2017) A comprehensive non-invasive framework for diagnosing prostate cancer. Comput Biol Med 81:148–158
Rubinstein E, Salhov M, Nidam-Leshem M, White V, Golan S, Baniel J, Bernstine H, Groshar D, Averbuch A (2019) Unsupervised tumor detection in dynamic PET/CT imaging of the prostate. Med Image Anal 55:27–40
Thomas S, Stephanie A, Evrim B, Deepak K, Sena T, Manuel M, Chris Y, Jonathan S, Sherif M, Pingkun Y, Sheng X, Bradford J, Maria J, Peter A, Peter L, Baris T (2020) Deep-learning-based artificial intelligence for PI-RADS classification to assist multiparametric prostate MRI interpretation: a development study. J Magn Reson Imaging 52(5):1499–1507
Jun A, Yoichiro Y, Tetsuro S, Yasushi N, Hiromu M, Kotaro T, Masato Y, Yuki E, Hayato T, Tatsuro H, Masao U, Gen T, Ichiro M, Manabu F, Akira S, Toyonori T, Go K (2019) illuminating clues of cancer buried in prostate MR image: deep learning and expert approaches. Biomolecules 9(11):673
Satheesh K, Krishna S, Nicola S, Fernando M, Benhabib H, Girish K, Matthew D, Ronaldo H (2020) Role of MRI in staging of penile cancer. J Magn Reson Imaging 51(6):1612–1629
Yang G, Wang C, Yang J, Chen Y, Tang L, Shao P, Dillenseger J-L, Shu H, Luo L (2020) Weakly-supervised convolutional neural networks of renal tumor segmentation in abdominal CTA images. BMC Med Imaging 20:20–37
Guanyu Y, Chuanxia W, Jian Y, Yang C, Lijun T, Pengfei S, Jean-Louis D, Huazhong S, Limin L (2020) Weakly-supervised convolutional neural networks of renal tumor segmentation in abdominal CTA images. BMC med imaging 20:11–37
Prathamesh M, Eric J, Jaya S, Robyn D-C, Bethany R, Megan H, Larisa J, Harriet M, Pok F, Balazs A, Emanuelle M, Chen Y, Manas M, Michael R (2020) Deep learning based on standard H&E images of primary melanoma tumors identifies patients at risk for visceral recurrence and death. Clin Cancer Res 26(5):1126–1134
Lindgren S, May S, Reza K, Olof E, Johannes U, Mads H, Jane S, Poul F-C, Lars E, Elin T (2019) Deep learning for segmentation of 49 selected bones in CT scans: first step in automated PET/CT-based 3D quantification of skeletal metastases. Eur J Radiol 113:89–95
Ruud J, Rogier R, Christophe K, Arnoud W, Maudy G, Harrie P, Georg S, Hessel W, Massimo M (2019) Deep learning for real-time, automatic, and scanner-adapted prostate (zone) segmentation of transrectal ultrasound, for example, magnetic resonance imaging–transrectal ultrasound fusion prostate biopsy. Eur urol focus 7(1):78–85
Arif M, Ivo G, Jose C, Chris H, Gabriel P, Monique J, Wiro N (2020) Clinically significant prostate cancer detection and segmentation in low-risk patients using a convolutional neural network on multi-parametric MRI. Eur Radiol. https://doi.org/10.1007/s00330-020-07008-z
Jae K, Mun J, Jong S, Jun H, Choung-Soo K, Seong I, Chang W, Seok-Soo B, Kyo C, Byung H, Yong H, Ji Y (2018) A Deep belief network and dempster-shafer-based multiclassifier for the pathology stage of prostate cancer. J Healthc Eng. https://doi.org/10.1155/2018/4651582
Lucas M, Jansen I, Savci-Heijink CD, Meijer SL, de Boer OJ, van Leeuwen TG, de Bruin DM, Marquering HA (2019) Deep learning for automatic Gleason pattern classification for grade group determination of prostate biopsies. Virchows Arch 475:77–83
Eirini P, May S, Reza K, Pablo B, Olof E, Johannes U, Mattias O, Elin T, Mads H, Jane A, Poul F-C, Åse A, Lars E (2019) Deep learning-based quantification of PET/CT prostate gland uptake: association with overall survival. Wiley
Ohad K, Drew L, Ali A, Andreas K, Carleen J, Dragan G, Thomas S, Boris G (2019) Development of a deep learning algorithm for the histopathologic diagnosis and gleason grading of prostate cancer biopsies: a pilot study. Eur Urol Focus. https://doi.org/10.1016/j.euf.2019.11.003
Yoichiro Y, Toyonori T, Jun A, Masao U, Hiromu M, Yasushi N, Taishi T, Takuji T, Kotaro T, Ryuto N, Akira S, Ichiro M, Shinichi T, Hiroyuki K, Yukihiro K (2019) Automated acquisition of explainable knowledge from unannotated histopathology images. Nat Commun 10(1):1–9
Amogh H, Rakesh S, Harri M, Prateek P, Otto E, Pekka T, Hannu J, Peter J, Ivan J, Anant M (2020) Test-retest repeatability of a deep learning architecture in detecting and segmenting clinically significant prostate cancer on apparent diffusion coefficient (ADC) maps. Eur Radiol. https://doi.org/10.1007/s00330-020-07065-4
Patrick S, Xianfeng W, Jan P, Manuel W, Philipp K, Albrecht S, Markus H, Heinz-Peter S, Klaus H-H (2020) Simulated clinical deployment of fully automatic deep learning for clinical prostate MRI assessment. Eur radiol 31:1–12
Ström P, Kartasalo K, Olsson H, Solorzano L, Delahunt B, Berney D, Bostwick D, Evans A, Grignon D, Humphrey P, Iczkowski K (2020) Artificial intelligence for diagnosis and grading of prostate cancer in biopsies: a population-based, diagnostic study. Lancet Oncol 21(2):222–232
Zhang H, Ji J, Liu Z, Lu H, Qian C, Wei C, Chen S, Lu W, Wang C, Xu H, Xu Y (2023) Artificial intelligence for the diagnosis of clinically significant prostate cancer based on multimodal data: a multicenter study. BMC Med 21(1):270
Bashashati A, Goldenberg S (2022) AI for prostate cancer diagnosis: hype or today’s reality? Nature Rev Urol 19(5):261–262
Bulten WEA (2022) Artificial intelligence for diagnosis and Gleason grading of prostate cancer: the PANDA challenge. Nature Med 28:154–163
Rho M, Park J, Moon H, Kim C, Jeon S, Kang M, Lee J (2022) Dr. Answer AI for prostate cancer: intention to use, expected effects, performance, and concerns of urologists. Prostate Int 10(1):38–44
Rodrigo S-I, Simon H, Gerd R, Christian G, Arkadiusz M (2020) Current and future applications of machine and deep learning in urology: a review of the literature on urolithiasis, renal cell carcinoma, and bladder and prostate cancer. World J Urol 38(10):2329–2347. https://doi.org/10.1007/s00345-019-03000-5
Milap S, Nithesh N, Bhaskar S, H. BM, (2020) Artificial intelligence (AI) in urology-current use and future directions: An iTRUE study. Turk J Urol 46:27–39. https://doi.org/10.5152/tud.2020.20117
Misgana N, Rodrigo S, Simon H, Arkadiusz M, Alexander R (2020) Application of artifcial neural networks for automated analysis of cystoscopic images: a review of the current status and future prospects. World J Urol 38:2349–2358. https://doi.org/10.1007/s00345-019-03059-0
Checcucci E, Autorino R, Cacciamani GE, Amparore D, De Cillis S, Piana A, Piazzolla P, Vezzetti E, Fiori C, Veneziano D, Tewari A (2020) Artificial intelligence and neural networks in urology: current clinical applications. Minerva Urol Nefrol 72(1):49–57
Funding
Not Applicable.
Ethics declarations
Conflict of interest
The authors state they have no known conflicting commercial interests or personal relationships that could have influenced this article’s work.
Human and animal rights
The authors did not perform any experiments with animals for conducting this research.
Informed consent
There is not any patient data used in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lubbad, M., Karaboga, D., Basturk, A. et al. Machine learning applications in detection and diagnosis of urology cancers: a systematic literature review. Neural Comput & Applic 36, 6355–6379 (2024). https://doi.org/10.1007/s00521-023-09375-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-023-09375-2