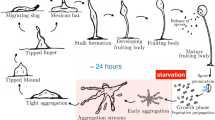
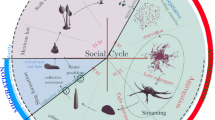
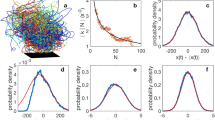
Abstract
Collective behaviour in nature provides a source of inspiration for engineering artificial systems (e.g. robotics, ecosystems of services), due to their inherent mechanisms favouring adaptation to environmental changes and enabling complex emergent behaviour to arise from a relatively simple behaviour of individual entities. The first-order emergence, also referred to as swarm intelligence, is well studied, while higher order levels of emergent behaviour have not received much attention yet. Second-order emergent behaviour arises from the interactions of individuals, which are themselves the result of first-order emergent behaviour. Dictyostelium discoideum provides a compelling case for studying both first- and second-order emergence. Individual cells move around on their own when there is plenty of food. When food is scarce, cells self-aggregate towards a leading center cell (first-order emergent behaviour) to build a super-organism, similar to a slug. The slug displays properties that none of the cells has on its own (e.g. sensitivity to light and heat). It moves as a whole (second-order emergent behaviour) looking for a suitable place to transform into a fruiting body (also known as sporocarp), where later the cells resume their individual behaviour. This paper focuses specifically on the aggregation and migration phases of Dictyostelium discoideum. We present two agent-based models, implemented in Matlab for first order and Python for second order. They display a series of emergent properties, among others homogeneous aggregation territories size (first order) and merging of slugs or new property as sensitivity to light (second order). Future works involve implementing and experimenting both first- and second-order emergence in swarm robotics, and identification of design patterns for engineering higher order emergent behaviour in artificial systems.









Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Notes
The surface is doubtless the best place from which to disperse spores to new environments that are well furnished with abundant bacterial food supplies.
DIF: differentiation inducing factor
References
Nagano S (2000) Modeling the model organism Dictyostelium discoideum. Dev Growth Differ 42(6):541–550
Vasieva O, Vasiev B (2013) Mathematical modeling in developmental biology. Reproduction 145(6):R175–R184
Strmecki L, Greene DM, Pears CJ (2005) Developmental decisions in Dictyostelium discoideum. Dev Biol 284(1):25–36
Bakthavatsalam D, Gomer RH (2010) The secreted proteome profile of developing Dictyostelium discoideum cells. Proteomics 10(13):2556–2559
Li SI, Purugganan MD (2011) The cooperative amoeba: Dictyostelium as a model for social evolution. Trends Gene 27(2):48–54
Agnew DJG, Green JEF, Brown TM, Simpson MJ, Binder BJ (2014) Distinguishing between mechanisms of cell aggregation using pair-correlation functions. J Theor Biol 352:16–23
McDonald SA (1986) Cell-cycle regulation of center initiation in Dictyostelium discoideum. Dev Biol 117(2):546–549
Glazer PM, Newell PC (1981) Initiation of aggregation by Dictyostelium discoideum in mutant populations lacking pulsatile signalling. Microbiology 125(2):221–232
Maeda Y (2005) Regulation of growth, differentiation in Dictyostelium. Int Rev Cytol 244:287–332
Mahadeo DC, Parent CA (2006) Signal relay during the life cycle of Dictyostelium. Curr Topics Dev Biol 73:115–140
Robison G (2012) Cyclic Amp. Elsevier, New York
Kessin RH (2001) Dictyostelium: evolution, cell biology, and the development of multicellularity. Cambridge University Press, Cambridge
Vinet AF, Fiedler T, Studer V, Froquet R, Dardel A, Cosson P, Pieters J (2014) Initiation of multicellular differentiation in Dictyostelium discoideum is regulated by coronin A. Mol Biol Cell 25(5):688–701
Mackay SA (1978) Computer simulation of aggregation in Dictyostelium discoideum. J Cell Sci 33(1):1–16
Loomis W (2012) Dictyostelium discoideum: a developmental system. Elsevier, Amsterdam
Raman RK, Hashimoto YOHICHI, Cohen MH, Robertson A (1976) Differentiation for aggregation in the cellular slime molds: the emergence of autonomously signaling cells in Dictyostelium discoideum. J Cell Sci 21(2):243–259
Vasieva OO, Vasiev BN, Karpov VA, Zaikin AN (1994) A model of Dictyostelium discoideum aggregation. J Cell Sci 171:361–367
Müller-Taubenberger A, Kortholt A, Eichinger L (2013) Simple system-substantial share: the use of Dictyostelium in cell biology, molecular medicine. Eur J Cell Biol 92(2):45–53
Lee SK, Yu SL, Alexander H, Alexander S (1998) A mutation in repB, the Dictyostelium homolog of the human xeroderma pigmentosum B gene, has increased sensitivity to UV-light but normal morphogenesis. Biochim Biophys Acta (BBA)-Gene Struct Express 1399(2–3):161–172
Wessels D, Srikantha T, Yi S, Kuhl S, Aravind L, Soll DR (2006) The Shwachman-Bodian-Diamond syndrome gene encodes an RNA-binding protein that localizes to the pseudopod of Dictyostelium amoebae during chemotaxis. J Cell Sci 119(2):370–379
Langenick J, Araki T, Yamada Y, Williams JG (2008) A Dictyostelium homologue of the metazoan Cbl proteins regulates STAT signaling. J Cell Sci 121(21):3524–3530
Li G, Alexander H, Schneider N, Alexander S (2000) Molecular basis for resistance to the anticancer drug cisplatin in Dictyostelium. Microbiology 146(9):2219–2227
Williams RSB, Eames M, Ryves WJ, Viggars J, Harwood AJ (1999) Loss of a prolyl oligopeptidase confers resistance to lithium by elevation of inositol (1, 4, 5) trisphosphate. EMBO J 18(10):2734–2745
Williams JG (2010) Dictyostelium finds new roles to model. Genetics 185(3):717–726
Harris E, Wang N, Wu W, Weatherford A, De Lozanne A, Cardelli J (2002) Dictyostelium LvsB mutants model the lysosomal defects associated with Chediak-Higashi syndrome. Mol Biol Cell 13(2):656–669
Grove JE, Brown RJ, Watts DJ (2000) The intracellular target for the antiresorptive aminobisphosphonate drugs in Dictyostelium discoideum is the enzyme farnesyl diphosphate synthase. J Bone, Miner Res 15(5):971–981
Sussman M, Noeël ELIZABETH (1952) An analysis of the aggregation stage in the development of the slime molds, Dictyosteliaceae. I. The populational distribution of the capacity to initiate aggregation. Biol Bull 103(2):259–268
Gomer RH, Jang W, Brazill D (2011) Cell density sensing and size determination. Dev Growth Differ 53(4):482–494
Van Oss C, Panfilov AV, Hogeweg P, Siegert F, Weijer CJ (1996) Spatial pattern formation during aggregation of the slime mold Dictyostelium discoideum. J Theor Biol 181(3):203–213
Fukujin F, Nakajima A, Shimada N, Sawai S (2016) Self-organization of chemoattractant waves in Dictyostelium depends on F-actin and cell–substrate adhesion. J Royal Soc Interface 13(119):20160233
Newell PC, Ross FM (1982) Inhibition by adenosine of aggregation centre initiation, cyclic AMP binding in Dictyostelium. Microbiology 128(11):2715–2724
Parent CA, Devreotes PN (1999) A cell’s sense of direction. Microbiology 284:765–770
Levchenko A, Iglesias PA (2002) Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys J 82(1):50–63
Krishnan J, Iglesias PA (2003) Analysis of the signal transduction properties of a module of spatial sensing in eukaryotic chemotaxis. Biophys J 65(1):95–128
Parhizkar M, Di Marzo SG (2017) An agent-based model for collective behaviours of social amoeba Dictyostelium discoideum Morphogenesis: aggregation phase. SWARM 2017 the 2nd international symposium on swarm behaviour and bio-inspired robotics
Becker M (2010) A simulation study of mechanisms of group selection of the slime mold Dictyostelium discoideum. In: 2010 IEEE 14th International Conference on Intelligent Engineering Systems IEEE, pp 321–326
Dallon J, Jang W, Gomer RH (2006) Mathematically modelling the effects of counting factor in Dictyostelium discoideum. Math Med Biol J IMA 23(1):45–62
Dallon JC, Dalton B, Malani C (2011) Understanding streaming in Dictyostelium discoideum: theory versus experiments. Bull Math Biol 73:1603–1626
Mogilner A (2009) Mathematics of cell motility: have we got its number? J Math Biol 58:105–134
Durston A (1974) Pacemaker mutants of Dictyostelium discoideum. Dev Biol 38:308–319
Weijer CJ, Williams JG (2001) Dictyostelium: cell Sorting and Patterning. Encyclopedia of life sciences (ELS). Wiley, New York
Kay RR, Large S, Traynor D, Nayler O (1993) A localized differentiation-inducing-factor sink in the front of the Dictyostelium slug. Proc Natl Acad Sci USA 90:487–491
Weijer CJ (2004) Dictyostelium morphogenesis. Curr Opin Genet Dev 14:392–398
Meng Y, Kazeem O, Muller JC (2007) A Swarm Intelligence Based Coordination Algorithm for Distributed Multi-Agent Systems. Integr Knowl Intensive Multi-Agent Syst 10:294–299
Fisher PR, Annesley SJ (2006) Slug phototaxis, thermotaxis, and spontaneous turning behaviour. In: Dictyostelium discoideum Protocols. Humana Press, pp 137–170
Srinivasan S, Alexander H, Alexander S (2000) The Dictyostelium fruiting body–a structure of cells and cellulose. Trends Cell Biol 10(8):315
Supriya S, Alexander H, Alexander S (2000) The Dictyostelium fruiting body-a structure of cells and cellulose. Trends Cell Biol 10:315
Acknowledgements
This work is supported by the Swiss National Science Foundation (SNSF) [Grant number 205321 179023].
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented in part at the 2nd International Symposium on Swarm Behavior and Bio-Inspired Robotics, Kyoto, October 29–November 1, 2017.
About this article
Cite this article
Parhizkar, M., Di Marzo Serugendo, G. Agent-based models for first- and second-order emergent collective behaviours of social amoeba Dictyostelium discoideum aggregation and migration phases. Artif Life Robotics 23, 498–507 (2018). https://doi.org/10.1007/s10015-018-0477-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10015-018-0477-3