Abstract
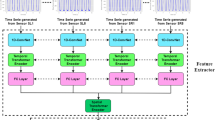
Clinical diagnosis of Parkingson’s disease (PD) requires the physician to assess the patient’s gait and other symptoms. A dual-branch model is proposed in this paper as an objective diagnostic tool to diagnose PD automatically. In this research, the joint features and independent features of left and right gait are fused innovatively. Convolutional neural network (CNN) and long short-term memory network (LSTM) are used to extract the spatial and temporal characteristics of sensors respectively. After the independent features extracted from the branches are collapsed, LSTM is used to incorporate the joint features between the left and right gait. Compared with other methods, the proposed model can learn the correlation between the two feet and extract higher discriminative features to effectively improve the accuracy of Parkinson detection. The model shows the state-of-the-art performance for the public dataset, with the accuracy, sensitivity, and specificity being 99.22%, 100%, and 98.04%, respectively. A simple, fast, and objective method proposed in this paper was believed to improve diagnostic performance.






Similar content being viewed by others
References
De Lau LML, Breteler MMB (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5(6):525–535
Mcgregor MM, Nelson AB (2019) Circuit mechanisms of Parkinson’s disease. Neuron 101 (6):1042–1056
Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson’s disease. J Neurochem 139:318–324
Marinus J, Zhu K, Marras C, Aarsland D, Van Hilten JJ (2018) Risk factors for non-motor symptoms in Parkinson’s disease 17(6):559–568
Tolosa E, Wenning GK, Poewe W (2006) The diagnosis of Parkinson’s disease. Lancet Neurol 5(1):75–8
Schrag A, Benshlomo Y, Quinn NP (2002) How valid is the clinical diagnosis of Parkinson’s disease in the community? J Neurol Neurosurg Psychiatr 73(5):529–534
Ali L, Zhu C, Zhou M, Liu Y (2019) Early diagnosis of Parkinson’s disease from multiple voice recordings by simultaneous sample and feature selection. Expert Syst Appl 137:22–28
Chen HL, Wang G, Ma C, Cai Z N, Liu WB, Wang SJ (2016) An efficient hybrid kernel extreme learning machine approach for early diagnosis of Parkinson’s disease. Neurocomputing 184:131–144
Gupta D, Julka A, Jain S, Aggarwal T, Khanna A, Arunkumar N, De Albuquerque VHC (2018) Optimized cuttlefish algorithm for diagnosis of Parkinson’s disease. Cognit Syst Res 52:36–48
Drotar P, Mekyska J, Rektorova I, Masarova L, Smekal Z, Faundezzanuy M (2016) Evaluation of handwriting kinematics and pressure for differential diagnosis of Parkinson’s disease. Artif Intell Med 67:39–46
Rovini E, Maremmani C, Cavallo F (2020) A wearable system to objectify assessment of motor tasks for supporting Parkinson’s disease diagnosis. Sensors 20(9):2630
Amoroso N, La Rocca M, Monaco A, Bellotti R, Tangaro S (2018) Complex networks reveal early MRI markers of Parkinson’s disease. Med Image Anal 12
Butt A H, Rovini E, Fujita H, Maremmani C, Cavallo F (2020) Data-driven models for objective grading improvement of Parkinson’s disease. Ann Biomed Eng 48(12):2976–2987
Alam N, Garg A, Munia TTK, Fazelrezai R, Tavakolian K (2017) Vertical ground reaction force marker for Parkinson’s disease. PLOS One 12(5):0175951–0175951
Asuroglu T, Acici K, Erdas CB, Toprak MK, Erdem H, Ogul H (2018) Parkinson’s disease monitoring from gait analysis via foot-worn sensors. Biocybern Biomed Eng 38(3):760– 772
Zhao A, Qi L, Li J, Dong J, Yu H (2018) A hybrid spatio-temporal model for detection and severity rating of Parkinson’s disease from gait data. Neurocomputing 315:1–8
Xia Y, Yao Z, Ye Q, Cheng N (2020) A dual-modal attention-enhanced deep learning network for quantification of Parkinson’s disease characteristics. IEEE Trans Neural Syst Rehabil Eng 28(1):42–51
Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM (2005) Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci 22(5):1248–1256
Hausdorff J M, Lowenthal J, Herman T, Gruendlinger L, Peretz C, Giladi N (2007) Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. Eur J Neurosci 26(8):2369–2375
Frenkeltoledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM (2005) Treadmill walking as an external pacemaker to improve gait rhythm and stability in Parkinson’s disease. Mov Disord 20(9):1109–111
Post B, Merkus MP, Bie RMAD, Haan RJD, Speelman JD (2005) Unified Parkinson’s disease rating scale motor examination: are ratings of nurses, residents in neurology, and movement disorders specialists interchangeable? Mov Disord 20(12):1577–1584
El Maachi I, Bilodeau G-A, Bouachir W (2020) Deep 1D-Convnet for accurate Parkinson disease detection and severity prediction from gait. Expert Syst Appl 113075:143
Hochreiter S, Schmidhuber J (1997) Long short-term memory. Neural Comput 9(8):1735–1780
Su B, Song R, Guo L, Yen C-W (2015) Characterizing gait asymmetry via frequency sub-band components of the ground reaction force. Biomed Signal Process Control 18:56–60
Rovini E, Maremmani C, Moschetti A, Esposito D, Cavallo F (2018) Comparative motor pre-clinical assessment in Parkinson’s disease using supervised machine learning approaches. Ann Biomed Eng 46 (12):2057–2068
Haixiang G, Yijing L, Shang J, Mingyun G, Yuanyue H, Bing G (2017) Learning from class-imbalanced data: review of methods and applications. Expert Syst Appl 73:220–239
Hausdorff JM (2009) Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos 19(2):2277–2332
Abdulhay E, Arunkumar N, Narasimhan K, Vellaiappan E, Venkatraman V (2018) Gait and tremor investigation using machine learning techniques for the diagnosis of Parkinson disease. Future Gener Comput Syst 83:366–373
Ren P, Karahan E, Chen C, Luo R, Valdes-Sosa PA (2017) Gait influence diagrams in Parkinson’s Disease. IEEE Trans Neural Syst Rehabil Eng PP(99):1–1
Zhang X, Yang Y, Li T, Zhang Y, Wang H, Fujita H (2020) CMC: a consensus multi-view clustering model for predicting Alzheimer’s disease progression. Comput Methods Progr Biomed 199: 105895
Funding
This work was supported by the Project of Supporting Plan of Tianjin (China) (16-YFZCSY00850).
Author information
Authors and Affiliations
Contributions
Xu Liu: Formal Analysis, Methodology, Data curation, Writing- Original draft. Wang Li: Logical combing, Theoretical analysis. Zheng Liu: Resources. Feixiang Du: Software. Qiang Zou: Conceptualization, Supervision, Writing- Reviewing & Editing.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Availability of data and material
The datasets used during the current study are available in the PhysioNet.
Code availability
According to the introduction of the paper, the model can be easily reproduced through TensorFlow library. If necessary, please contact the corresponding author to explain the purpose . If the reason is right, we will reply to the download link of code.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Li, W., Liu, Z. et al. A dual-branch model for diagnosis of Parkinson’s disease based on the independent and joint features of the left and right gait. Appl Intell 51, 7221–7232 (2021). https://doi.org/10.1007/s10489-020-02182-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10489-020-02182-5