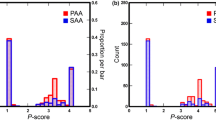
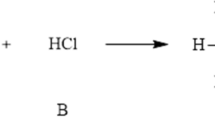
Summary
The mutagenic activity of 23 triazenes and, in a different set, of 24 halogenated hydroxyfuranones (MX derivatives) is quantitatively related to new features of contemporary molecular wave functions. Nowadays affordable computers are powerful enough to rapidly generate geometry-optimised ab initio wave functions at HF/3-21G*, HF/6-31G* and B3LYP/6-311+G(2d,p) level for all molecules. The bonds of a common molecular skeleton are described by their ab initio bond lengths and local properties provided by the theory of quantum chemical topology (QCT). The chemometric analysis involves two types: one to generate a statistically validated quantitative model, and one to isolate the active center. In the former a genetic algorithm (GA) selects bond descriptors in order to optimise the cross-validation error, q2, followed by a full partial least squares (PLS) analysis, which also yields randomisation statistics. In the latter type principal components (PCs) are constructed from the original bond descriptors and their variables important to the projection (VIPs) are plotted in a histogram. This analysis suggests a preferred mechanistic pathway for the initial hydroxylation of the triazenes, an issue that has remained ambiguous so far. In the case of the hydroxyfuranones the proposed method aids the elucidation of a mechanistic ambivalence.
Similar content being viewed by others
References
D. Voet J.G. Voet (1995) Biochemistry EditionNumber2 Wiley New York, USA
D. Maron B.N. Ames (1983) Mutat. Res., 113 173
S.E. O’Brien P.L.A. Popelier (2001) J. Chem. Inf. Comp. Sci., 41 764
A.J. Shusterman A.K. Debnath C. Hansch W.H. Gregory R.F. Frank A.C. Greene S.F. Watkins (1989) Mol. Pharm., 36 939
K. Tuppurainen S. Lotjonen (1993) Mutat. Res., 287 235
R.F.W. Bader (1990) Atoms in Molecules. A Quantum Theory Oxford University Press Oxford, UK
R.F.W. Bader (1985) Acc. Chem. Res., 18 9
R.F.W. Bader (1991) Chem. Rev., 91 893
P.L.A. Popelier (2000) Atoms in Molecules. An Introduction Pearson Education London, UK
P.L.A. Popelier (1999) J. Phys. Chem. A, 103 2883
S.E. O’Brien P.L.A. Popelier (2002) J. Chem. Soc. Perkin Trans. 2 478
P.J. Smith P.L.A. Popelier (2004) J. Comput.-Aided Mol. Des., 18 135
O’Brien, S.E. and Popelier, P.L.A., ECCOMAS, Barcelona, Spain, 2000.
P.L.A. Popelier U. Chaudry P.J. Smith (2002) J. Chem. Soc. Perkin Trans. 2 1231
U.A. Chaudry P.L.A. Popelier (2003) J. Phys. Chem. A, 107 4578
U.A. Chaudry P.L.A. Popelier (2004) J. Org. Chem., 69 233
P.L.A. Popelier (1994) Chem. Phys. Lett., 228 160
N.O.J. Malcolm P.L.A. Popelier (2002) J. Comp. Chem., 24 437
R.F.W. Bader T.S. Slee D. Cremer E. Kraka (1983) J. Am. Chem. Soc., 105 5061
S.T. Howard O. Lamarche (2003) J. Phys. Org. Chem., 16 133
J. Cioslowski S.T. Mixon (1991) J. Am. Chem. Soc., 113 4142
R.F.W. Bader T.T. Nguyen-Dang Y. Tal (1981) Rep. Prog. Phys., 44 893
J.R. Cheeseman M.T. Carroll R.F.W. Bader (1988) Chem. Phys. Lett., 143 450
R.F.W. Bader H.J.T. Preston (1969) Int. J. Quant. Chem., 3 327
G. Schaftenaar J.H. Noordik (2000) J. Comput.-Aided Mol. Des., 14 123
GAUSSIAN98. Gaussian 98, Revision A.7, Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Zakrzewski, V. G. Montgomery, J. A., Jr., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J. M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Baboul, A.G., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Gonzalez, C., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Andres, J.L., Gonzalez, C., Head-Gordon, M., Replogle, E.S. and Pople, J.A., Gaussian, Inc., Pittsburgh, PA, USA, 1998.
M.J.S. Dewar E.G. Zoebisch E.F. Healy J.J.P. Stewart (1985) J. Am. Chem. Soc., 107 3902
J.B. Foresman A. Frisch (1996) Exploring Chemistry with Electronic Structure Methods Gaussian Inc. Pittsburgh, PA USA
A.D. Becke (1993) J. Chem. Phys., 98 5648
MORPHY98. a program written by P.L.A. Popelier with a contribution from R.G.A. Bone, UMIST, Manchester, UK (1998). http://morphy.ch.umist.ac.uk/.
Popelier, P.L.A., Chaudry, U.A. and Smith, P.J., J. Chem. Soc., Perkin Trans. II (2002) 1231.
Wold, S., Sjostrom, M. and Eriksson, L., In Schleyer, P., Encycl. of Comp. Chem. Wiley, Chichester, UK, 1998, p. 2006.
S. Wold N. Kettaneh K. Tjessem (1996) J. Chemometr., 10 463
J.H. Holland (1992) Adaption in Natural and Artificial Systems MIT Press Cambridge, MA, USA
UMETRICS. info@umetrics.com: www.umetrics.com, 2002.
D.J. Livingstone (1995) Data Analysis for Chemists Oxford University Press Oxford, UK
H.H. Maw L.H. Hall (2001) J. Chem. Inf. Comput. Sci., 41 1248
B.M. Wise N.B. Gallagher (2003) Eigenvector Research Manson WA, USA
Wold, S., In van de Waterbeemd, H. (Ed.) Chemometric Methods in Molecular Design, VCH, Weinheim, 1995, p.195.
O’Brien, S.E., Dept. of Chemistry, UMIST, Manchester, UK, 2000.
SPSS Inc. version 10.0.7 http://www.spss.com, 2000 ,Chicago, IL ,USA
B.H. Venger C. Hansch G.J. Hatheway Y.U. Amrein (1979) J. Med. Chem., 22 473
A.J. Shusterman A.S. Johnson C. Hansch (1989) Int. J. Quant. Chem., 36 19
C. Hansch (1972) Drug Metab. Rev., 1 1
J.M. Pires W.B. Floriano A.C. Gaudio (1997) J. Mol. Struct., 389 159
K. Fukui T. Yonezawa C. Nagata (1952) J. Chem. Phys., 20 722
M. Ho H. Schmider K.E. Edgecombe V.H. Smith (1994) J. Int. J. Quant. Chem. Quant. Chem. Symp., 28 215
Popelier, P.L.A., In Ford, M., Livingstone, D.J., Dearden, J. and van de Waterbeemd, H. (Eds.), EuroQSAR2002: Designing Drugs and Crop Protectants: Processes, Problems and Solutions. Blackwell, Oxford, UK, 2003, p. 130.
R.T. LaLonde G.P. Cook H. Perakyla L. Bu (1992) Chem. Res. Toxicol., 4 540
K. Tuppurainen (1999) Chemosphere, 38 3015
K. Tupparainen S. Lotjonen R. Lattikainen T. Vartiainen U. Maran M. Strandberg T. Tamm (1991) Mutat. Res., 247 97
K. Tupparainen S. Lotjonen R. Laatikainen T. Vartiainen (1992) Mutat. Res., 266 181
A. Poso K. Tuppurainen J. Gynther (1994) J. Mol. Struct.-Theochem., 304 255
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popelier, P.L.A., Smith, P.J. & Chaudry, U.A. Quantitative structure-activity relationships of mutagenic activity from quantum topological descriptors: triazenes and halogenated hydroxyfuranones (mutagen-X) derivatives. J Comput Aided Mol Des 18, 709–718 (2004). https://doi.org/10.1007/s10822-004-6815-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-004-6815-7