Summary
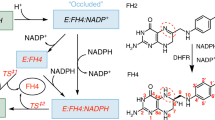
R67 dihydrofolate reductase (DHFR), a bacterial plasmid-encoded enzyme associated with resistance to the drug trimethoprim, shows neither sequence nor structural homology with the chromosomal DHFR. It presents a highly symmetrical toroidal structure, where four identical monomers contribute to the unique central active-site pore. Two reactants (dihydrofolate, DHF), two cofactors (NADPH) or one of each (R67•DHF•NADPH) can be found simultaneously within the active site, the last one being the reactive ternary complex. As the positioning of the ligands has proven elusive to empirical determination, we addressed the problem from a theoretical perspective. Several potential structures of the ternary complex were generated using the docking programs AutoDock and FlexX. The variability among the final poses, many of which conformed to experimental data, prompted us to perform a comparative scoring analysis and molecular dynamics simulations to assess the stability of the complexes. Analysis of ligand–ligand and ligand–protein interactions along the 4 ns trajectories of eight different structures allowed us to identify important inter-ligand contacts and key protein residues. Our results, combined with published empirical data, clearly suggest that multipe binding modes of the ligands are possible within R67 DHFR. While the pterin ring of DHF and the nicotinamide ring of NADPH assume a stacked endo-conformation at the centre of the pore, probably assisted by V66, Q67 and I68, the tails of the molecules extend towards opposite ends of the cavity, adopting multiple configurations in a solvent rich-environment where hydrogen-bond interactions with K32 and Y69 may play important roles.
Similar content being viewed by others
Abbreviations
- R67 DHFR:
-
R67 dihydrofolate reductase
- DHF:
-
dihydrofolate
- DHFH+:
-
N5- protonated dihydrofolate
- NADPH:
-
reduced nicotinamide adenine dinucleotide phosphate
- NMN:
-
nicotinamide-ribose-phosphate moiety of NADPH
- pte:
-
pterin ring of folate
- nic:
-
nicotinamide ring of NADPH
- pABA-Glu:
-
para-aminobenzoyl glutamic acid tail of folate
- 2′,5′-ADP:
-
adenosine diphosphate ribose moiety of NADPH
References
K.H. Pattishall J. Acar J.J. Burchall F.W. Goldstein R.J. Harvey (1977) J. Biol. Chem. 252 2319 Occurrence Handle1:CAS:528:DyaE2sXhvVGjs7o%3D Occurrence Handle14961
J.F. Acar F.W. Goldstein G.R. Gerbaud Y.A. Chabbert (1977) Ann. Microbiol. (Paris) 128A 41 Occurrence Handle1:STN:280:CSiB3c%2FhsFM%3D
J.W. Zolg U.J. Hanggi (1981) Nucl. Acids Res. 9 697 Occurrence Handle1:CAS:528:DyaL3MXkslKjtL8%3D Occurrence Handle6261228
P. Radstrom O. Skold G. Swedberg J. Flensburg P.H. Roy L. Sundstrom (1994) J. Bacteriol. 176 3257 Occurrence Handle1:STN:280:ByuB2M3pvFA%3D Occurrence Handle8195081
T.M. L’Abee-Lund H. Sorum (2001) Microbiol. Drug Resist 7 263 Occurrence Handle10.1089/10766290152652819 Occurrence Handle1:CAS:528:DC%2BD3MXpt1Wjt7s%3D
F.M. Huennekens (1996) Protein Sci. 5 1201 Occurrence Handle1:CAS:528:DyaK28XjsVKgurY%3D Occurrence Handle8762155
C.A. Fierke K.A. Johnson S.J. Benkovic (1987) Biochemistry 26 4085 Occurrence Handle10.1021/bi00387a052 Occurrence Handle1:CAS:528:DyaL2sXktF2qs7c%3D Occurrence Handle3307916
M.R. Sawaya J. Kraut (1997) Biochemistry 36 586 Occurrence Handle10.1021/bi962337c Occurrence Handle1:CAS:528:DyaK2sXmtVymsA%3D%3D Occurrence Handle9012674
M. Grape L. Sundstrom G. Kronvall (2003) Microbiol. Drug Resist 9 317 Occurrence Handle10.1089/107662903322762734 Occurrence Handle1:CAS:528:DC%2BD2cXhtVKktbs%3D
P.T. Rajagopalan S. Lutz S.J. Benkovic (2002) Biochemistry 41 12618 Occurrence Handle10.1021/bi026369d Occurrence Handle1:CAS:528:DC%2BD38Xntlaitrs%3D Occurrence Handle12379104
R.M. Brito R. Reddick G.N. Bennett F.B. Rudolph P.R. Rosevear (1990) Biochemistry 29 9825 Occurrence Handle10.1021/bi00494a011 Occurrence Handle1:CAS:528:DyaK3cXlvVOjtLw%3D Occurrence Handle2271620
C. Bystroff S.J. Oatley J. Kraut (1990) Biochemistry 29 3263 Occurrence Handle10.1021/bi00465a018 Occurrence Handle1:CAS:528:DyaK3cXhsFChu7s%3D Occurrence Handle2185835
T.D. Bradrick J.M. Beechem E.E. Howell (1996) Biochemistry 35 11414 Occurrence Handle10.1021/bi960205d Occurrence Handle1:CAS:528:DyaK28XkvVGqsbc%3D Occurrence Handle8784197
N. Narayana D.A. Matthews E.E. Howell X. Nguyen-huu (1995) Nat. Struct. Biol. 2 1018 Occurrence Handle10.1038/nsb1195-1018 Occurrence Handle1:CAS:528:DyaK2MXptlegtrc%3D Occurrence Handle7583655
H. Park P. Zhuang R. Nichols E.E. Howell (1997) J. Biol. Chem. 272 2252 Occurrence Handle10.1074/jbc.272.4.2252 Occurrence Handle1:CAS:528:DyaK2sXotV2msQ%3D%3D Occurrence Handle8999931
D. Li L.A. Levy S.A. Gabel M.S. Lebetkin E.F. DeRose M.J. Wall E.E. Howell R.E. London (2001) Biochemistry 40 4242 Occurrence Handle10.1021/bi0026425 Occurrence Handle1:CAS:528:DC%2BD3MXhvVSms70%3D Occurrence Handle11284680
S.N. Hicks R.D. Smiley J.B. Hamilton E.E. Howell (2003) Biochemistry 42 10569 Occurrence Handle10.1021/bi034643d Occurrence Handle1:CAS:528:DC%2BD3sXmsFamsrw%3D Occurrence Handle12962480
R.M. Brito F.B. Rudolph P.R. Rosevear (1991) Biochemistry 30 1461 Occurrence Handle10.1021/bi00220a003 Occurrence Handle1:CAS:528:DyaK3MXnslGksw%3D%3D Occurrence Handle1993165
W.H., Pitcher Suffix3rd E.F. DeRose G.A. Mueller E.E. Howell R.E. London (2003) Biochemistry 42 11150 Occurrence Handle10.1021/bi0349874 Occurrence Handle1:CAS:528:DC%2BD3sXmvVGgt74%3D Occurrence Handle14503865
S.N. Hicks R.D. Smiley L.G. Stinnett K.H. Minor E.E. Howell (2004) J. Biol. Chem. 279 46995 Occurrence Handle10.1074/jbc.M404484200 Occurrence Handle1:CAS:528:DC%2BD2cXptFejsL4%3D Occurrence Handle15333636
E.E. Howell U. Shukla S.N. Hicks R.D. Smiley L.A. Kuhn M.I. Zavodszky (2001) J. Comput. Aided Mol. Des. 15 1035 Occurrence Handle10.1023/A:1014824725891 Occurrence Handle1:CAS:528:DC%2BD38Xjt1Cjsbg%3D Occurrence Handle11989624
M.B. Strader R.D. Smiley L.G. Stinnett N.C. VerBerkmoes E.E. Howell (2001) Biochemistry 40 11344 Occurrence Handle10.1021/bi0110544 Occurrence Handle1:CAS:528:DC%2BD3MXmt1Olt70%3D Occurrence Handle11560482
T.J.A. Ewing I.D. Kuntz (1997) J. Comput. Chem. 18 1175 Occurrence Handle10.1002/(SICI)1096-987X(19970715)18:9<1175::AID-JCC6>3.0.CO;2-O Occurrence Handle1:CAS:528:DyaK2sXksVKmsLs%3D
V. Schnecke C.A. Swanson E.D. Getzoff J.A. Tainer L.A. Kuhn (1998) Proteins 33 74 Occurrence Handle10.1002/(SICI)1097-0134(19981001)33:1<74::AID-PROT7>3.0.CO;2-L Occurrence Handle1:CAS:528:DyaK1cXlvFCisLw%3D Occurrence Handle9741846
R.D. Smiley L.G. Stinnett A.M. Saxton E.E. Howell (2002) Biochemistry 41 15664 Occurrence Handle10.1021/bi026676j Occurrence Handle1:CAS:528:DC%2BD38Xpt1yntrY%3D Occurrence Handle12501195
L.G. Stinnett R.D. Smiley S.N. Hicks E.E. Howell (2004) J. Biol. Chem. 279 47003 Occurrence Handle10.1074/jbc.M404485200 Occurrence Handle1:CAS:528:DC%2BD2cXptFejsL8%3D Occurrence Handle15333637
M.B. Strader S. Chopra M. Jackson R.D. Smiley L. Stinnett J. Wu E.E. Howell (2004) Biochemistry 43 7403 Occurrence Handle10.1021/bi049646k Occurrence Handle1:CAS:528:DC%2BD2cXktVKlsLw%3D Occurrence Handle15182183
G.M. Morris D.S. Goodsell R.S. Halliday R. Huey W.E. Hart R.K. Belew A.J. Olson (1998) J. Comput. Chem. 19 1639 Occurrence Handle10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B Occurrence Handle1:CAS:528:DyaK1cXntFemur4%3D
B. Kramer M. Rarey T. Lengauer (1999) Proteins 37 228 Occurrence Handle10.1002/(SICI)1097-0134(19991101)37:2<228::AID-PROT8>3.0.CO;2-8 Occurrence Handle1:CAS:528:DyaK1MXmvFWrsrs%3D Occurrence Handle10584068
M. Kontoyianni L.M. McClellan G.S. Sokol (2004) J.␣Med. Chem. 47 558 Occurrence Handle10.1021/jm0302997 Occurrence Handle1:CAS:528:DC%2BD2cXjt1Gk Occurrence Handle14736237
E. Kellenberger J. Rodrigo P. Muller D. Rognan (2004) Proteins 57 225 Occurrence Handle10.1002/prot.20149 Occurrence Handle1:CAS:528:DC%2BD2cXnvFaku7k%3D Occurrence Handle15340911
T. Schulz-Gasch M. Stahl (2003) J. Mol. Model (Online) 9 47 Occurrence Handle1:CAS:528:DC%2BD3sXivFyisbY%3D
B.D. Bursulaya M. Totrov R. Abagyan C.L., Brooks Suffix3rd (2003) J. Comput. Aided Mol. Des. 17 755 Occurrence Handle10.1023/B:JCAM.0000017496.76572.6f Occurrence Handle1:CAS:528:DC%2BD2cXhsFOhtL0%3D Occurrence Handle15072435
R.D. Taylor P.J. Jewsbury J.W. Essex (2002) J. Comput. Aided Mol. Des. 16 151 Occurrence Handle10.1023/A:1020155510718 Occurrence Handle1:CAS:528:DC%2BD38XotFekurg%3D Occurrence Handle12363215
R. Wang Y. Lu S. Wang (2003) J. Med. Chem. 46 2287 Occurrence Handle10.1021/jm0203783 Occurrence Handle1:CAS:528:DC%2BD3sXjsVOgtr0%3D Occurrence Handle12773034
P. Ferrara H. Gohlke D.J. Price G. Klebe C.L., Brooks Suffix3rd (2004) J. Med. Chem. 47 3032 Occurrence Handle10.1021/jm030489h Occurrence Handle1:CAS:528:DC%2BD2cXjs1Sjsrk%3D Occurrence Handle15163185
F. Cheng E. Oldfield (2004) J. Med. Chem. 47 5149 Occurrence Handle10.1021/jm040036s Occurrence Handle1:CAS:528:DC%2BD2cXntlOjtL0%3D Occurrence Handle15456258
W.M. Rockey A.H. Elcock (2002) Proteins 48 664 Occurrence Handle10.1002/prot.10186 Occurrence Handle1:CAS:528:DC%2BD38XmslWjsLc%3D Occurrence Handle12211034
C. Mulakala P.J. Reilly (2002) Proteins 49 125 Occurrence Handle10.1002/prot.10206 Occurrence Handle1:CAS:528:DC%2BD38XntVygs7w%3D Occurrence Handle12211022
W.M. Brown D.L. Vander Jagt (2004) J. Chem. Inform. Comput. Sci. 44 1412 Occurrence Handle10.1021/ci049853r Occurrence Handle1:CAS:528:DC%2BD2cXksVGqsLg%3D
B. Kramer M. Rarey T. Lengauer (1999) Proteins 37 228 Occurrence Handle10.1002/(SICI)1097-0134(19991101)37:2<228::AID-PROT8>3.0.CO;2-8 Occurrence Handle1:CAS:528:DyaK1MXmvFWrsrs%3D Occurrence Handle10584068
M. Rarey B. Kramer T. Lengauer G. Klebe (1996) J.␣Mol. Biol. 261 470 Occurrence Handle10.1006/jmbi.1996.0477 Occurrence Handle1:CAS:528:DyaK28XltlKisLo%3D Occurrence Handle8780787
R.J. Rosenfeld D.S. Goodsell R.A. Musah G.M. Morris D.B. Goodin A.J. Olson (2003) J. Comput. Aided Mol. Des. 17 525 Occurrence Handle10.1023/B:JCAM.0000004604.87558.02 Occurrence Handle1:CAS:528:DC%2BD3sXptVOkt7c%3D Occurrence Handle14703123
INSIGHT II. 1998 Biosym/MSI: San Diego, CA
C. Hetenyi D. Spoel Particlevan der (2002) Protein Sci. 11 1729 Occurrence Handle10.1110/ps.0202302 Occurrence Handle1:CAS:528:DC%2BD38XltVyns7w%3D Occurrence Handle12070326
H.J. Bohm (1994) J. Comput. Aided Mol. Des. 8 243 Occurrence Handle10.1007/BF00126743 Occurrence Handle1:STN:280:ByqD2Mvit1M%3D Occurrence Handle7964925
M. Rarey B. Kramer T. Lengauer (1999) Proteins 34 17 Occurrence Handle10.1002/(SICI)1097-0134(19990101)34:1<17::AID-PROT3>3.0.CO;2-1 Occurrence Handle1:CAS:528:DyaK1MXjtFaqug%3D%3D Occurrence Handle10336380
H.J.C. Berendsen D. Vanderspoel R. Vandrunen (1995) Comput. Phys. Commun. 91 43 Occurrence Handle10.1016/0010-4655(95)00042-E Occurrence Handle1:CAS:528:DyaK2MXps1Wrtr0%3D
E. Lindahl B. Hess D. Spoel Particlevan der (2001) J. Mol. Mod. 7 306 Occurrence Handle1:CAS:528:DC%2BD3MXnsVGjsL4%3D
H. Gohlke M. Hendlich G. Klebe (2000) J. Mol. Biol. 295 337 Occurrence Handle10.1006/jmbi.1999.3371 Occurrence Handle1:CAS:528:DC%2BD3cXht1Crtw%3D%3D Occurrence Handle10623530
R.D. Clark A. Strizhev J.M. Leonard J.F. Blake J.B. Matthew (2002) J. Mol. Graph Model 20 281 Occurrence Handle10.1016/S1093-3263(01)00125-5 Occurrence Handle1:CAS:528:DC%2BD3MXpt1art7g%3D Occurrence Handle11858637
P.S. Charifson J.J. Corkery M.A. Murcko W.P. Walters (1999) J. Med. Chem. 42 5100 Occurrence Handle10.1021/jm990352k Occurrence Handle1:CAS:528:DyaK1MXnsFOqt7g%3D Occurrence Handle10602695
G.A. Kaminski R.A. Friesner J. Tirado-Rives W.L. Jorgensen (2001) J. Phys. Chem. B 105 6474 Occurrence Handle10.1021/jp003919d Occurrence Handle1:CAS:528:DC%2BD3MXislKhsLk%3D
W.L. Jorgensen J. Tirado-Rives (1988) J. Am. Chem. Soc. 110 1657 Occurrence Handle10.1021/ja00214a001 Occurrence Handle1:CAS:528:DyaL1cXht1yjt7Y%3D
H.J.C. Berendensen J.P.M. Postma W.F. van Gunsteren J. Hermans (1981) Interactions Models for Water in Relation to Protein Hydration D. Reidel Publishing Company Dordrecht 331–342
M.A. Kastenholz P.H. Hunenberger (2004) J. Phys. Chem. B 108 774 Occurrence Handle10.1021/jp0350924 Occurrence Handle1:CAS:528:DC%2BD3sXpvVSntLo%3D
H. Deng R. Callender E. Howell (2001) J. Biol. Chem. 276 48956 Occurrence Handle10.1074/jbc.M105107200 Occurrence Handle1:CAS:528:DC%2BD38Xktlegsg%3D%3D Occurrence Handle11679579
Hicks, S.N., Smiley, R.D., Stinnett, L.G., Minor, K.H. and Howell, E.E., J. Biol. Chem. 279 (2004) 46995
R. Castillo J. Andres V. Moliner (1999) J. Am. Chem. Soc. 121 12140 Occurrence Handle10.1021/ja9843019 Occurrence Handle1:CAS:528:DyaK1MXnvVOmtr8%3D
F. Iribarne M. Paulino S. Aguilera M. Murphy O. Tapia (2002) J. Mol. Model (Online) 8 173 Occurrence Handle10.1007/s00894-002-0082-0 Occurrence Handle1:CAS:528:DC%2BD38XmtFWjt7k%3D
A. Cavalli G. Bottegoni C. Raco M. Vivo ParticleDe M. Recanatini (2004) J. Med. Chem. 47 3991 Occurrence Handle10.1021/jm040787u Occurrence Handle1:CAS:528:DC%2BD2cXkvFyhtL8%3D Occurrence Handle15267237
N. Moitessier C. Henry B. Maigret Y. Chapleur (2004) J.␣Med. Chem. 47 4178 Occurrence Handle10.1021/jm0311386 Occurrence Handle1:CAS:528:DC%2BD2cXlvVSkt78%3D Occurrence Handle15293990
V. Zoete M. Meuwly M. Karplus (2004) Proteins 55 568 Occurrence Handle10.1002/prot.20071 Occurrence Handle1:CAS:528:DC%2BD2cXjvFemt7g%3D Occurrence Handle15103621
J. Choe S. Suresh G. Wisedchaisri K.J. Kennedy M.H. Gelb W.G.J. Hol (2002) Chem. Biol. 9 1189 Occurrence Handle10.1016/S1074-5521(02)00243-0 Occurrence Handle1:CAS:528:DC%2BD38XoslGntLk%3D Occurrence Handle12445769
W.E. Minke J. Pickens E.A. Merritt E.K. Fan C.L.M.J. Verlinde W.G.J. Hol (2000) Acta Crystallogr. D-Biol. Crystallogr. 56 795 Occurrence Handle10.1107/S090744490000514X Occurrence Handle10930826
P.J. Lewi M. Jonge Particlede F. Daeyaert L. Koymans M. Vinkers J. Heeres P.A. Janssen E. Arnold K. Das A.D. Clark SuffixJr. S.H. Hughes P.L. Boyer M.P. Bethune Particlede R. Pauwels K. Andries M. Kukla D. Ludovici B. Corte ParticleDe R. Kavash C. Ho P.J. Lewis (2003) J.␣Comput. Aided Mol. Des. 17 129 Occurrence Handle10.1023/A:1025313705564 Occurrence Handle13677481
K.S. Bak-Jensen G. Andre T.E. Gottschalk G. Paes V. Tran B. Svensson (2004) J. Biol. Chem. 279 10093 Occurrence Handle10.1074/jbc.M312825200 Occurrence Handle1:CAS:528:DC%2BD2cXhvFCitb4%3D Occurrence Handle14660599
T.P. Ko R. Williams A. McPherson (1996) Acta Crystallogr. D-Biol. Crystallogr. 52 160 Occurrence Handle10.1107/S0907444995009127 Occurrence Handle1:STN:280:DC%2BD2czpsFWhsQ%3D%3D Occurrence Handle15299737
P.R. Thompson J. Schwartzenhauer D.W. Hughes A.M. Berghuis G.D. Wright (1999) J. Biol. Chem. 274 30697 Occurrence Handle10.1074/jbc.274.43.30697 Occurrence Handle1:CAS:528:DyaK1MXntVSmt7Y%3D Occurrence Handle10521458
H. Park T.D. Bradrick E.E. Howell (1997) Protein Eng. 10 1415 Occurrence Handle10.1093/protein/10.12.1415 Occurrence Handle1:CAS:528:DyaK1cXitVWhs70%3D Occurrence Handle9543003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alonso, H., Gillies, M.B., Cummins, P.L. et al. Multiple ligand-binding modes in bacterial R67 dihydrofolate reductase. J Comput Aided Mol Des 19, 165–187 (2005). https://doi.org/10.1007/s10822-005-3693-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10822-005-3693-6