Summary
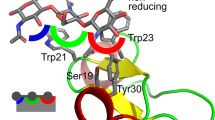
Aromatic amino acid residues are often present in carbohydrate-binding sites of proteins. These binding sites are characterized by a placement of a carbohydrate moiety in a stacking orientation to an aromatic ring. This arrangement is an example of CH/π interactions. Ab initio interaction energies for 20 carbohydrate–aromatic complexes taken from 6 selected ultra-high resolution X-ray structures of glycosidases and carbohydrate-binding proteins were calculated. All interaction energies of a pyranose moiety with a side chain of an aromatic residue were calculated as attractive with interaction energy ranging from −2.8 to −12.3 kcal/mol as calculated at the MP2/6-311+G(d) level. Strong attractive interactions were observed for a wide range of orientations of carbohydrate and aromatic ring as present in selected X-ray structures. The most attractive interaction was associated with apparent combination of CH/π interactions and classical H-bonds. The failure of Hartree–Fock method (interaction energies from +1.0 to −6.9 kcal/mol) can be explained by a dispersion nature of a majority of the studied complexes. We also present a comparison of interaction energies calculated at the MP2 level with those calculated using molecular mechanics force fields (OPLS, GROMOS, CSFF/CHARMM, CHEAT/CHARMM, Glycam/AMBER, MM2 and MM3). For a majority of force fields there was a strong correlation with MP2 values. RMSD between MP2 and force field values were 1.0 for CSFF/CHARMM, 1.2 for Glycam/AMBER, 1.2 for GROMOS, 1.3 for MM3, 1.4 for MM2, 1.5 for OPLS and to 2.3 for CHEAT/CHARMM (in kcal/mol). These results show that molecular mechanics approximates interaction energies very well and support an application of molecular mechanics methods in the area of glycochemistry and glycobiology.
Similar content being viewed by others
Abbreviations
- AMBER:
-
assisted model building with energy refinement
- B3LYP:
-
Becke–Slater-HF 3-term exchange and Lee–Yang–Parr correlation hybrid functional
- BSSE:
-
basis set superposition error
- CBM:
-
carbohydrate-binding module
- CBS:
-
complete basis set
- CCSD(T):
-
coupled cluster with single, double and perturbative triple excitation
- CHARMM:
-
chemistry at Harvard molecular mechanics
- CHEAT:
-
carbohydrate hydroxyl groups represented by extended atoms
- CSFF:
-
carbohydrate solution force field
- DFT:
-
density functional theory
- GROMOS:
-
Groningen molecular simulation
- HF:
-
Hartree–Fock␣method
- MM2:
-
molecular mechanics version 2
- MM3:
-
molecular mechanics version 3
- MP2:
-
Møller–Plesset perturbation theory; second order
- OPLS:
-
optimized potentials for liquid simulations
- PDB:
-
protein data bank
- RMSD:
-
root mean square deviation
References
Hobza P., Zahradník R., 1980. Weak Intermolecular Interactions in Chemistry and Biology Elsevier Scientific Pub. Co. Amsterdam, New York
Nishio M., Hirota M., Umezawa Y., 1998. The CH/π Interaction: Evidence, Nature, and Consequences John Wiley & Sons New York
Tamres M., (1952) J. Am. Chem. Soc. 74: 3375
Hobza P., Havlas Z., (2000) Chem. Rev. 100: 4253
Brandl M., Weiss M.S., Jabs A., Sühnel J., Hilgenfeld R., (2001) J. Mol. Biol. 307: 357
Takahashi O., Kohno Y., Iwasaki S., Saito K., Iwaoka M., Tomoda S., Umezawa Y., Tsuboyama S., Nishio M., (2001) Bull. Chem. Soc. Jpn. 74: 2421
Nishio M., (2004) Cryst. Eng. Comm. 6: 130
Tsuzuki S., Honda K., Uchimaru T., Mikami M., Tanabe K., (2000) J. Am. Chem. Soc. 122: 3746
Tarakeshwar P., Choi H.S., Kim K.S., (2001) J. Am. Chem. Soc. 123: 3323
Bagno A., Saielli G., Scorrano G., (2002) Chem. Eur. J. 8: 2047
Karlstrom G., Linse P., Wallqvist A., Jonssonf B., (1983) J. Am. Chem. Soc. 105: 3717
Hobza P., Selzle H.L., Schlag E.W., (1994) J. Am. Chem. Soc. 116: 3500
Hobza P., Selzle H.L., Schlag E.W., (1996) J. Phys. Chem. 100: 18790
Tsuzuki S., Uchimaru T., Matsumura K., Mikami M., Tanabe K., (2000) Chem. Phys. Lett. 319: 547
Hobza P., Spirko V., Havlas Z., Buchhold K., Reimann B., Barth H.-D., Brutschy B., (1999) Chem. Phys. Lett. 299: 180
Muraki M., (2002) Protein Pept. Lett.9: 195
Sujatha M.S., Balaji B.V., (2004) Proteins55: 44
Muraki M., Harata K., (2000) Biochemistry39: 292
Huber R.E., Hakda S., Cheng C., Cupples C.G., Edwards R.A., (2003) Biochemistry42: 1796
Zolotnitsky G., Cogan U., Adir N., Solomon V., Shoham G., Shoham Y., (2004) Proc. Natl. Acad. Sci. USA 101: 11275
Davis A.P., Wareham R.S., (1999) Angew. Chem. Int. Ed. Engl. 38: 2978
Spiwok V., Lipovová P., Skálová T., Buchtelová E., Hašek J., Králová B., (2004) Carbohydr. Res. 339: 2275
Sujatha M.S., Sasidhar Y.U., Balaji P.V., (2004) Protein Sci.13: 2502
Sujatha M.S., Sasidhar Y.U., Balaji P.V., (2005) Biochemistry44: 8554
del Carmen Fernandez-Alonso M., Cañada F.J., Jiménez-Barbero J., Cuevas G., (2005) J. Am. Chem. Soc. 127: 7379
Damm W., Frontera A., Tirado-Rives J., Jorgensen W.L., (1997) J. Comp. Chem. 18: 1955
Kuttel M., Brady J.W., Naidoo K.J., (2002) J. Comput. Chem. 23: 1236
Kouwijzer M.L.C.E., Grootenhuis P.D.J., (1995) J. Phys. Chem. 99: 13426
Woods R.J., Dwek R.A., Edge C.J., Fraser-Reid B., (1995) J. Phys. Chem. 99: 3832
Pérez S., Imberty A., Engelsen S.B., Gruza J., Mazeau K., Jiménez-Barbero J., Poveda A., Espinosa J.-F., van Eyck B.P., Johnson G.J., et al., (1998) Carbohydr. Res. 314: 141
Hemmingsen L., Madsen D.E., Esbensen A.L., Olsen L., Engelsen S.B., (2004) Carbohydr. Res. 339: 937
Kubinyi, H., In Testa, B., van de Waterbeemd, H., Folkers, G. and Guy, R., (Eds.), Pharmacokinetic Optimization in Drug Research. Biological, Physicochemical, and Computational Strategies, Helvetica Chimica Acta and Wiley-VCH, Zürich, 2001, pp. 513–524
Ladbury J.E., (1996) Chem. Biol. 3: 973
Kellenberger E., Rodrigo J., Muller P., Rognan D., (2004) Proteins57: 225
Møller C., Plesset M.S., (1934) Phys. Rev. 46: 618
Lütteke T., Frank M., von der Lieth C.W., (2004) Carbohydr. Res. 339: 1015
Guérin D.M.A., Lascombe M.-B., Costabel M.B., Souchon H., Lamzin V., Béguin P., Alzari P.M., (2002) J. Mol. Biol. 316: 1061
Sanders D.A.R., Moothoo D.N., Raftery J., Howard A.J., Helliwell J.R., Naismith J.H., (2001) J. Mol. Biol. 310: 875
Merritt E.A., Kuhn P., Sarfaty S., Erbe J.L., Holmes R.K., Hol W.G.J., (1998) J. Mol. Biol. 282: 1043
Notenboom V., Boraston A.B., Williams J.S., Kilburn D.G., Rose D.R., (2002) Biochemistry 41: 4246
Varrot A., Frandsen T.B., von Ossowski I., Boyer V., Cottaz S., Driguez H., Schülein M., Davies G.J., (2003) Structure 11: 855
Charnock S.J., Bolam D.N., Nurizzo D., Szabó L., McKie V.A., Gilbert H.J., Davies G.J., (2002) Proc. Natl. Acad. Sci. USA 99: 14077
Schmidt M.W., Baldridge K.K., Boatz J.A., Elbert S.T., Gordon M.S., Jensen J.H., Koseki S., Matsunaga N., Nguyen K.A., Su S., Windus T.L., Dupuis M., Montgomery J.A., (1993) J. Comput. Chem. 14: 1347
Boys S.F., Bernardi F., (1970) Mol. Phys. (UK) 19: 553
Jorgensen W.L., Tirado-Rives J., (1988) J. Am. Chem. Soc. 110: 1657
van Gunsteren, W.F., Billeter, S., Eising, A.A., Hübenberger, P.H., Krüger, P., Mark, A.E., Scott, W.R.P. and Tironi, I.G., Biomolecular Simulations: The GROMOS 96 Manual and User Guide VDF Zürich, 1996
MacKerell Jr., A.D., Bashford D., Bellott M., Dunbrack Jr. R.L., Evanseck J.D., Field M.J., Fischer S., Gao J., Guo H., Ha S., Joseph-McCarthy D., Kuchnir L., Kuczera K., Lau F.T.K., Mattos C., Michnick S., Ngo T., Nguyen D.T., Prodhom B., Reiher III., W.E., Roux B., Schlenkrich M., Smith J.C., Stote R., Straub J., Watanabe M., Wiorkiewicz-Kuczera J., Yin D., Karplus M., (1998) J. Phys. Chem. B. 102: 3586
Pearlman D.A., Case D.A., Caldwell J.W., Ross W.R., Cheatham T.E., DeBolt III., S., Ferguson D., Seibel G., Kollman P., (1995) Comp. Phys. Commun. 91: 1
Allinger N.L., Kok R.A., Imam M.R., (1988) J. Comput. Chem.9: 591
Lii J.-H., Allinger N.L., (1989) J. Am. Chem. Soc. 111: 8576
Pappu R.V., Hart R.K., Ponder J.W., (1998) J. Phys. Chem. B. 102: 9725
Guex N., Peitsch M.C., (1997) Electrophoresis 18: 2714
Turnbull W.B., Precious B.L., Homans S.W., (2004) J Am Chem Soc. 126: 1047
Coutinho, P.M. and Henrissat, B., Carbohydrate-Active Enzymes server, 1999: http://afmb.cnrs-mrs.fr/CAZY/
Coutinho, P.M. and Henrissat, B., In Gilbert, H.J., Davies, G., Henrissat, B. and Svensson, B. (Eds.), Recent Advances in Carbohydrate Bioengineering. The Royal Society of Chemistry, Cambridge, 1999, pp. 3–12
Johnson E.R., Wolkow R.A., DiLabio G.A., (2004) Chem. Phys. Lett. 394: 334
Grimme S., (2004) J. Comput. Chem. 25: 1463
Morales J.C., Penadés S., (1998) Angew. Chem. Int. Edit. 37: 654
Acknowledgements
Authors would like to gratefully acknowledge the Czech Science Foundation (GACR 204/02/0843) and the Academy of Sciences of the Czech Republic (projects B500500512 and AVOZ 40500505) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spiwok, V., Lipovová, P., Skálová, T. et al. Modelling of carbohydrate–aromatic interactions: ab initio energetics and force field performance. J Comput Aided Mol Des 19, 887–901 (2005). https://doi.org/10.1007/s10822-005-9033-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-005-9033-z