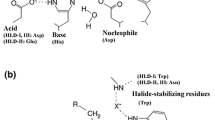
Abstract
1,2,3-Trichloropropane (TCP) is a highly toxic, recalcitrant byproduct of epichlorohydrin manufacture. Haloalkane dehalogenase (DhaA) from Rhodococcus sp. hydrolyses the carbon–halogen bond in various halogenated compounds including TCP, but with low efficiency (k cat/K m = 36 s-1 M-1). A Cys176Tyr-DhaA mutant with a threefold higher catalytic efficiency for TCP dehalogenation has been previously obtained by error-prone PCR. We have used molecular simulations and quantum mechanical calculations to elucidate the molecular mechanisms involved in the improved catalysis of the mutant, and enantioselectivity of DhaA toward TCP. The Cys176Tyr mutation modifies the protein access and export routes. Substitution of the Cys residue by the bulkier Tyr narrows the upper tunnel, making the second tunnel “slot” the preferred route. TCP can adopt two major orientations in the DhaA enzyme, in one of which the halide-stabilizing residue Asn41 forms a hydrogen bond with the terminal halogen atom of the TCP molecule, while in the other it bonds with the central halogen atom. The differences in these binding patterns explain the preferential formation of the (R)- over the (S)-enantiomer of 2,3-dichloropropane-1-ol in the reaction catalyzed by the enzyme.




Similar content being viewed by others
References
Tesoriero AJ, Loffler FE, Liebscher H (2001) Environ Sci Technol 35:455–461
Dolfing J, Janssen DB (1994) Biodegradation 5:21–28
Swanson PE (1999) Curr Opin Biotechnol 10:365–369
Verschueren KHG, Seljee F, Rozeboom HJ, Kalk KH, Dijkstra BW (1993) Nature 363:693–698
Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, Sussman JL, Verschueren KHG, Goldman A (1992) Protein Eng 5:197–211
Newman J, Peat TS, Richard R, Kan L, Swanson PE, Affholter JA, Holmes IH, Schindler JF, Unkefer CJ, Terwilliger TC (1999) Biochemistry 38:16105–16114
Bosma T, Damborsky J, Stucki G, Janssen DB (2002) Appl Environ Microbiol 68:3582–3587
Janssen DB (2004) Curr Opin Chem Biol 8:150–159
Guex N, Peitsch MC (1997) Electrophoresis 18:2714–2723
Vriend G (1990) J Mol Graph 8:52–56
Goodford PJ (1985) J Med Chem 28:849–857
Otyepka M, Damborsky J (2002) Protein Sci 11:1206–1217
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) J Am Chem Sci 117:5179–5197
Cornell WD, Cieplak P, Bayly CI, Kollman PA (1993) J Am Chem Soc 115:9620–9631
Frisch MJ, Frisch A, Foresman JB (1998) Gaussian 98. Gaussian Inc., Pittsburgh
Case DA, Pearlman DA, Caldwell JW, Cheatham TE III, Ross WS, Simmerling CL, Darden TA, Merz KM, Stanton RV, Cheng AL, Vincent JJ, Crowley M, Tsui V, Radmer RJ, Duan Y, Pitera J, Seibel GL, Singh UC, Weiner PK, Kollman PA (1999) V. AMBER 6.0. University of California, San Francisco
Mahalanobis PC (1936) Proc Natl Inst Sci India 2:49–55
Lightstone FC, Zheng YJ, Bruice TC (1998) J Am Chem Soc 120:5611–5621
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) J Am Chem Soc 107:3902–3909
Damborsky J, Prokop M, Koca J (2001) Trends Biochem Sci 26:71–73
Prokop M, Damborsky J, Koca J (2000) Bioinformatics 16:845–846
Stewart JJP (1990) J Comput Aided Mol Des 4:1–45
Cernohorsky M, Kuty M, Koca J (1996) Comput Chem 21:35–44
Petrek M, Otyepka M, Banas P, Kosinova P, Koca J, Damborsky J (2006) BMC Bioinformatics 7:316
Bosma T, Pikkemaat MG, Kingma J, Dijk J, Janssen DB (2003) Biochemistry 42:8047–8053
Oakley A, Prokop Z, Bohac M, Kmunicek J, Jedlicka T, Monincova M, Kuta-Smatanova I, Nagata Y, Damborsky J, Wilce MCJ (2002) Biochemistry 41:4847–4855
Marek M, Vevodova J, Kuta-Smatanova I, Nagata Y, Swensson LA, Newman J, Takagi M, Damborsky J (2000) Biochemistry 39:14082–14086
Streltsov VA, Prokop Z, Damborsky J, Nagata Y, Oakley A, Wilce MCJ (2003) Biochemistry 42:10104–10112
Chaloupkova R, Sykorova J, Prokop Z, Jesenska A, Monincova M, Pavlova M, Tsuda M, Nagata Y, Damborsky J (2003) J Biol Chem 278:52622–52628
Acknowledgments
We thank Dr Tjibe Bosma (Groningen, the Netherlands) for valuable discussions on the interpretation of kinetic characterizations of mutant dehalogenases. We acknowledge financial support from the Czech Ministry of Education (MO, grants MSM6198959216, LC512; JD, grant MSM0021622412). The research of JD is supported by an EMBO/HMMI grant within the Young Investigator Program. We thank Dr J. Blackwell (UK) for linguistic revisions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Banáš, P., Otyepka, M., Jeřábek, P. et al. Mechanism of enhanced conversion of 1,2,3-trichloropropane by mutant haloalkane dehalogenase revealed by molecular modeling. J Comput Aided Mol Des 20, 375–383 (2006). https://doi.org/10.1007/s10822-006-9071-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-006-9071-1