Abstract
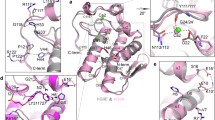
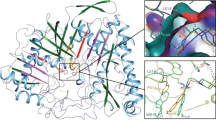
Docking calculations that allow the estimation of the binding energy of small ligands in the GIIA sPLA2 active site were used in a structure-based design protocol. Four GIIA sPLA2 inhibitors co-crystallised with the enzyme, were used for examining the enzyme active site and for testing the FlexX in SYBYL 6.8 molecular docking program to reproduce the crystallographic experimental data. The FPL67047XX inhibitor was chosen as a prototype structure for applying free energy perturbation (FEP) studies. Structural modifications of the initial structure of the FPL67047XX inhibitor (IC50 0.013 μM) were performed in an effort to optimise the interactions in the GIIA sPLA2 active site. The structural modifications were based on: (1) the exploration of absolute configuration (i.e. comparison of the binding score of (R)- and (S)-enantiomers); (2) bioisosterism (i.e. replacement of the carboxylate group with the bioisosteric sulphonate and phosphonate groups); (3) insertion of substituents that fit better in the active site. The generated new structures exhibited higher binding energy. Such structures may spark off the interest of medicinal chemists for synthesizing potentially more active GIIA sPLA2 inhibitors.




Similar content being viewed by others
References
Burke JE, Dennis EA (2009) Cardiovasc Drugs Ther 23:49–59
Schaloske RH, Dennis EA (2006) Biochim Biophys Acta 1761:1246–1259
Samuelsson B (1983) Science 220:568–575
Samuelsson B (1983) Adv Prostaglandin Thromboxane Leukot Res 11:1–13
Smith WL (1992) Am J Physiol 263:F181–F191
Tjoelker LW, Stafforini DM (2000) Biochim Biophys Acta 1488:102–123
Diaz BL, Arm JP (2003) Prostaglandins Leukot Essent Fatty Acids 69:87–97
Valentin E, Lambeau G (2000) Biochim Biophys Acta 1488:59–70
Lambeau G, Gelb MH (2008) Annu Rev Biochem 77:495–520
Boyanovsky BB, Webb NR (2009) Cardiovasc Drugs Ther 23:61–72
Seilhamer JJ, Pruzanski W, Vadas P, Plant S, Miller JA, Kloss J, Johnson LK (1989) J Biol Chem 264:5335–5338
WRJr Henderson, Chi EY, Bollinger JG, Tien YT, Ye X, Castelli L, Rubtsov YP, Singer AG, Chiang GK, Nevalainen T, Rudensky AY, Gelb MH (2007) J Exp Med 204:865–877
Rosenson RS (2009) Cardiovasc Drugs Ther 23:93–101
Oestvang J, Johansen B (2006) Biochim Biophys Acta 1761:1309–1316
Nevalainen TJ, Haapamaki MM, Gronroos JM (2000) Biochim Biophys Acta 1488:83–90
Kramer RM, Hession C, Johansen B, Hayes G, McGray P, Chow EP, Tizard R, Pepinsky RB (1989) J Biol Chem 264:5768–5775
Hara S, Kudo I, Chang HW, Matsuta K, Miyamoto T, Inoue K (1989) J Biochem 105:395–399
Lai FA, Anderson K, Rousseau E, Liu QY, Meissner G (1988) Biochem Biophys Res Commun 151:441–449
Bomalaski JS, Lawton P, Browning JL (1991) J Immunol 146:3904–3910
Scott DL, White SP, Browning JL, Rosa JJ, Gelb MH, Sigler PB (1991) Science 254:1007–1010
Wery JP, Schevitz RW, Clawson DK, Bobbitt JL, Dow ER, Gamboa G, TJr Goodson, Hermann RB, Kramer RM, McClure DB et al (1991) Nature 352:79–82
Cha SS, Lee D, Adams J, Kurdyla JT, Jones CS, Marshall LA, Bolognese B, Abdel-Meguid SS, Oh BH (1996) J Med Chem 39:3878–3881
Hagishita S, Yamada M, Shirahase K, Okada T, Murakami Y, Ito Y, Matsuura T, Wada M, Kato T, Ueno M, Chikazawa Y, Yamada K, Ono T, Teshirogi I, Ohtani M (1996) J Med Chem 39:3636–3658
Teshirogi I, Matsutani S, Shirahase K, Fujii Y, Yoshida T, Tanaka K, Ohtani M (1996) J Med Chem 39:5183–5191
Baba A, Kawamura N, Makino H, Ohta Y, Taketomi S, Sohda T (1996) J Med Chem 39:51765182
De Rosa M, Giordano S, Scettri A, Sodano G, Soriente A, Pastor PG, Alcaraz MJ, Paya M (1998) J Med Chem 41:3232–3238
Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, Sadilek M, Nguyen E, Lazdunski M, Lambeau G, Gelb MH (2002) J Biol Chem 277:48535–48549
Oslund RC, Cermak N, Gelb MH (2008) J Med Chem 51:4708–4714
Magrioti V, Kokotos G (2006) Antiinflamm Antiallergy Agents Med Chem 5:189–203
Reid RC (2005) Curr Med Chem 12:3011–3026
Meyer MC, Rastogi P, Beckett CS, McHowat J (2005) Curr Pharm Des 11:1301–1312
Garcia-Garcia HM, Serruys PW (2009) Curr Opin Lipidol 20:327–332
Magrioti V, Kokotos G (2010) Expert Opin Ther Pat 20:1–18
Draheim SE, Bach NJ, Dillard RD, Berry DR, Carlson DG, Chirgadze NY, Clawson DK, Hartley LW, Johnson LM, Jones ND, McKinney ER, Mihelich ED, Olkowski JL, Schevitz RW, Smith AC, Snyder DW, Sommers CD, Wery JP (1996) J Med Chem 39:5159–5175
Hansford KA, Reid RC, Clark CI, Tyndall JDA, Whitehouse MW, Guthrie T, McGeary RP, Schafer K, Martin JL, Fairlie DP (2003) Chembiochem 4:181–185
Bennion C, Connolly S, Gensmantel NP, Hallam C, Jackson CG, Primrose WU, Roberts GC, Robinson DH, Slaich PK (1992) J Med Chem 35:2939–2951
Jackson RC (1995) Curr Opin Biotechnol 6:646–651
Ortiz AR, Pisabarro MT, Gago F, Wade RC (1995) J Med Chem 38:2681–2691
Noel JP, Bingman CA, Deng TL, Dupureur CM, Hamilton KJ, Jiang RT, Kwak JG, Sekharudu C, Sundaralingam M, Tsai MD (1991) Biochemistry 30:11801–11811
Ortiz AR, Pisabarro MT, Gallego J, Gago F (1992) Biochemistry 31:2887–2896
Li B, Liu Z, Zhang L (2009) J Chem Inf Model 49:1725–1733
Tomoo K, Yamane A, Ishida T, Fujii S, Ikeda K, Iwama S, Katsumura S, Sumiya S, Miyagawa H, Kitamura K (1997) Biochim Biophys Acta 1340:178–186
Thunnissen MM, Kalk KH, Drenth J, Dijkstra BW (1990) J Mol Biol 216:425–439
Hariprasad V, Kulkarni VM (1996) J Mol Recognit 9:95–102
Halperin I, Ma B, Wolfson H, Nussinov R (2002) Proteins 47:409–443
Taylor RD, Jewsbury PJ, Essex JW (2002) J Comput Aided Mol Des 16:151–166
Tuccinardi T (2009) Comb Chem High Throughput Screen 12:303–314
Beaton HG, Bennion C, Connolly S, Cook AR, Gensmantel NP, Hallam C, Hardy K, Hitchin B, Jackson CG, Robinson DH (1994) J Med Chem 37:557–559
Kramer B, Rarey M, Lengauer T (1999) Proteins 37:228–241
Rarey M, Kramer B, Lengauer T (1999) Bioinformatics 15:243–250
Schevitz RW, Bach NJ, Carlson DG, Chirgadze NY, Clawson DK, Dillard RD, Draheim SE, Hartley LW, Jones ND, Mihelich ED et al (1995) Nat Struct Biol 2:458–465
Edwards SH, Thompson D, Baker SF, Wood SP, Wilton DC (2002) Biochemistry 41:15468–15476
Sybyl molecular modeling software packages, version 6.8., 2001, Tripos Inc., St. Louis, MO 63144
Clark M, Crammer DR III, Van Opdenbosch N (1989) J Comput Chem 10:982–1012
Powell DJM (1977) Math Program 12:241–254
Bohm HJ (1994) J Comput Aided Mol Des 8:243–256
Scott DL, White SP, Otwinowski Z, Yuan W, Gelb MH, Sigler PB (1990) Science 250:1541–1546
Burley SK, Petsko GA (1985) Science 229:23–28
Hunter CA, Singh J, Thornton JM (1991) J Mol Biol 218:837–846
Sinnokrot MO, Valeev EF, Sherrill CD (2002) J Am Chem Soc 124:10887–10893
Lima LM, Barreiro EJ (2005) Curr Med Chem 12:23–49
Patani GA, LaVoie EJ (1996) Chem Rev 96:3147–3176
Acknowledgments
Varnavas D. Mouchlis has been partially financed for this research by two bilateral agreements between Cyprus-Slovenia (CY-SLO/0407/06) and Cyprus-Romania (CY-ROM/0407/07) and part of this work was performed using CART facilities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mouchlis, V.D., Mavromoustakos, T.M. & Kokotos, G. Design of new secreted phospholipase A2 inhibitors based on docking calculations by modifying the pharmacophore segments of the FPL67047XX inhibitor. J Comput Aided Mol Des 24, 107–115 (2010). https://doi.org/10.1007/s10822-010-9319-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-010-9319-7