Abstract
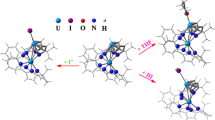
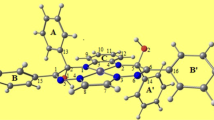
The potential energy surfaces of chiral tetraamine Pt(II) coordination complexes were computed at the B3LYP/LANL2DZ level of theory by a systematic variation of two dihedral angles: C12–C15–C34–C37 (θ) and C24–C17–C31–C48 (ψ) employing a grid resolution of 30°. Potential energy surfaces calculated using density functional theory methods and Boltzmann-derived populations revealed strong preference for one diasteromer of each series studied. In addition, natural bond orbital analysis show that the minima are stabilized predominantly by a combination of electronic interactions between two phenyl groups, the phenyl groups and the Pt2+ ion, as well as with the amine groups. Additional experimental characterization of the diasteroisomers studied here is in progress and will permit further molecular modeling studies with the appropriate stereochemistry.







Similar content being viewed by others
References
Sun RW-Y, Ma D-L, Wong EL-M, Che C-M (2007) Dalton Trans 43:4884–4892
Inouye Y, Kanamori T, Sugiyama M, Yoshida T, Koike T, Shionoya M, Enomoto K, Suehiro K, Kimura E (1995) Antivir Chem Chemother 6:337–344
Singh RV, Joshi SC, Kulshrestha S, Nagpal P, Bansal A (2001) Metal Based Drugs 8:149–158
Ming L-J, Epperson JD (2002) J Inorg Biochem 91:46–58
Hollis LS, Amundsen AR, Stern EW (1989) J Med Chem 32:128–136
Zheng H, Hu W, Yu D, Shen D-Y, Fu S, Kavanagh JJ, Wei I-C, Yang DJ (2008) Pharm Res 25:2272–2282
Meggers E (2007) Curr Opin Chem Biol 11:287–292
Orvig C, Abrams MJ (1999) Chem Rev 99:2201–2203
Bregman H, Carroll PJ, Meggers E (2006) J Am Chem Soc 128:877–884
Budzisz E, Malecka M, Lorenz I-P, Mayer P, Kwiecien RA, Paneth P, Krajewska U, Rozalski M (2006) Inorg Chem 45:9688–9695
Lovejoy KS, Todd RC, Zhang S, McCormick MS, D’Aquino JA, Reardon JT, Sancar A, Giacomini KM, Lippard SJ (2008) Proc Natl Acad Sci USA 105:8902–8907
Williams DS, Carroll PJ, Meggers E (2007) Inorg Chem 46:2944–2946
Rosenberg B, Van Camp L, Krigas L (1965) Nature 205:698–699
Rosenberg B (1985) Cancer 55:2303–2316
Ludwig T, Riethmuller C, Gekle M, Schwerdt G, Oberleithner H (2004) Kidney Int 66:196–202
Weiss RB, Christian MC (1993) Drugs 46:360–377
Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, Lippard SJ, Giacomini KM (2006) Cancer Res 66:8847–8857
Dooley CT, Chung NN, Wilkes BC, Schiller PW, Bidlack JM, Pasternak GW, Houghten RA (1994) Science 266:2019–2022
Houghten RA, Pinilla C, Appel JR, Blondelle sE, Dooley CT, Eichler J, Nefzi A, Ostresh JM (1999) J Med Chem 42:3743–3778
Pinilla C, Appel JR, Borras E, Houghten RA (2003) Nat Med 9:118–122
Nefzi A, Hoesl CE, Pinilla C, Kauffman GB, Maggiora GM, Pasquale E, Houghten RA (2006) J Comb Chem 8:780–783
Meggers E (2009) Chem Commun 1001–1010
Mora MA, Raya A, Mora-Ramirez MA (2002) Int J Quantum Chem 90:882–887
Hasinoff BB, Wu X, Yang Y (2004) J Inorg Biochem 98:616–624
Davies HO, Brown DA, Yanovsky AI, Nolan KB (1998) Inorg Chim Acta 268:313–316
Messori L, Shaw J, Camalli M, Mura P, Marcon G (2003) Inorg Chem 42:6166–6168
Granifo J, Vargas ME, Rocha H, Garland MT, Baggio R (2001) Inorg Chim Acta 321:209–214
Rotondo A (2006) Acta Crystallogr Sect C: Cryst Struct Commun 62:m19–m21
Hollis LS, Amundsen AR, Stern EW (1985) J Am Chem Soc 107:274–276
Liljefors T, Pettersson I (2002) In: Krogsgaard-Larsen P, Liljefors T, Madsen U (eds) Textbook of drug design and discovery. Taylor & Francis, London, pp 86–116
Perola E, Charifson PS (2004) J Med Chem 47:2499–2510
Butler KT, Luque FJ, Barril X (2009) J Comput Chem 30:601–610
Yongye AB, Foley BL, Woods RJ (2008) J Phys Chem A 112:2634–2639
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) Theoretical Chemistry Institute, University of Wisconsin, Madison: NBO 5.0
Dunning TH Jr, Hay PJ (1976) In: Schaefer HF III (ed) Modern theoretical chemistry. Plenum, New York, p 1
Hay PJ, Wadt WR (1985) J Chem Phys 82:270–283
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Wadt WR, Hay PJ (1985) J Chem Phys 82:284–298
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr. JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda J, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui A, Baboul AG, Clifford S, Cioslowshi J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004)
Dennington II R, Keith T, Millam J (2007) Semichem Inc., Shawnee Mission, KS
Acknowledgments
This work was supported by the State of Florida, Executive Officer of the Governor’s Office of Tourism, Trade and Economic Development, and by the National Science Foundation (CHE0455072 to R.A.H.). The authors thank the Florida State University High Performance Computing Facility for supercomputing time and Dr. Carmen Ortega-Alfaro for insightful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yongye, A.B., Giulianotti, M.A., Nefzi, A. et al. Conformational landscape of platinum(II)-tetraamine complexes: DFT and NBO studies. J Comput Aided Mol Des 24, 225–235 (2010). https://doi.org/10.1007/s10822-010-9328-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-010-9328-6