Abstract
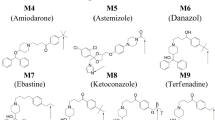
CYP2D6 is an important enzyme that is involved in first pass metabolism and is responsible for metabolizing ~25% of currently marketed drugs. A homology model of CYP2D6 was built using X-ray structures of ligand-bound CYP2C5 complexes as templates. This homology model was used in docking studies to rationalize and predict the site of metabolism of known CYP2D6 substrates. While the homology model was generally found to be in good agreement with the recently solved apo (ligand-free) X-ray structure of CYP2D6, significant differences between the structures were observed in the B′ and F–G helical region. These structural differences are similar to those observed between ligand-free and ligand-bound structures of other CYPs and suggest that these conformational changes result from induced-fit adaptations upon ligand binding. By docking to the homology model using Glide, it was possible to identify the correct site of metabolism for a set of 16 CYP2D6 substrates 85% of the time when the 5 top scoring poses were examined. On the other hand, docking to the apo CYP2D6 X-ray structure led to a loss in accuracy in predicting the sites of metabolism for many of the CYP2D6 substrates considered in this study. These results demonstrate the importance of describing substrate-induced conformational changes that occur upon binding. The best results were obtained using Glide SP with van der Waals scaling set to 0.8 for both the receptor and ligand atoms. A discussion of putative binding modes that explain the distribution of metabolic sites for substrates, as well as a relationship between the number of metabolic sites and substrate size, are also presented. In addition, analysis of these binding modes enabled us to rationalize the typical hydroxylation and O-demethylation reactions catalyzed by CYP2D6 as well as the less common N-dealkylation.












Similar content being viewed by others
References
Kola I, Landis J (2004) Opinion: can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 3:711–716
Danielson PB (2002) The cytochrome P 450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab 3:561–597
Auclair K, Hu Z, Little DM, Ortiz de Montellano PR, Groves JT (2002) Revisiting the mechanism of P450 enzymes with the radical clocks norcarane and spiro[2,5]octane. J Am Chem Soc 124:6020–6027
Bertz RJ, Granneman GR (1997) Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet 32:210–258
Bertilsson L, Dahl M-L, Dalen P, Al-Shurbaji A (2002) Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol 53:111–122
Strobl GR, von Kruedener S, Stoeckigt J, Guengerich FP, Wolff T (1993) Development of a pharmacophore for inhibition of human liver cytochrome P-450 2D6: molecular modeling and inhibition studies. J Med Chem 36:1136–1145
Michalets EL (1998) Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 18:84–112
Lennard MS (1990) Genetic polymorphism of sparteine/debrisoquine oxidation: a reappraisal. Pharmacol Toxicol 67:273–283
Cruciani G, Carosati E, De Boeck B, Ethirajulu K, Mackie C, Howe T, Vianello R (2005) MetaSite: understanding metabolism in human cytochromes from the perspective of the chemist. J Med Chem 48:6970–6979
Trunzer M, Faller B, Zimmerlin A (2009) Metabolic soft spot identification and compound optimization in early discovery phases using MetaSite and LC-MS/MS validation. J Med Chem 52:329–335
De Groot MJ, Ackland MJ, Horne VA, Alex AA, Jones BC (1999) A novel approach to predicting P450 mediated drug metabolism. CYP2D6 catalyzed N-dealkylation reactions and qualitative metabolite predictions using a combined protein and pharmacophore model for CYP2D6. J Med Chem 42:4062–4070
Hiroi T, Kishimoto W, Chow T, Imaoka S, Igarashi T, Funae Y (2001) Progesterone oxidation by cytochrome P450 2D isoforms in the brain. Endocrinology 142:3901–3908
Guengerich FP, Miller GP, Hanna IH, Martin MV, Leger S, Black C, Chauret N, Silva JM, Trimble LA, Yergey JA, Nicoll-Griffith DA (2002) Diversity in the oxidation of substrates by cytochrome P450 2D6: lack of an obligatory role of aspartate 301-substrate electrostatic bonding. Biochemistry 41:11025–11034
Yano JK, Hsu M-H, Griffin KJ, Stout CD, Johnson EF (2005) Structures of human microsomal cytochrome P450 2A6 complexed with coumarin and methoxsalen. Nat Struct Mol Biol 12:822–823
Yano JK, Wester MR, Schoch GA, Griffin KJ, Stout CD, Johnson EF (2004) The structure of human microsomal cytochrome P450 3A4 determined by X-ray crystallography to 2.05-Å resolution. J Biol Chem 279:38091–38094
Williams PA, Cosme J, Vinkovic DM, Ward A, Angove HC, Day PJ, Vonrhein C, Tickle IJ, Jhoti H (2004) Crystal structures of human cytochrome P450 3A4 bound to metyrapone and progesterone. Science 305:683–686
Wester MR, Johnson EF, Marques-Soares C, Dijols S, Dansette PM, Mansuy D, Stout CD (2003) Structure of mammalian cytochrome P450 2C5 complexed with diclofenac at 2.1 Å resolution: evidence for an induced fit model of substrate binding. Biochemistry 42:9335–9345
Wester MR, Johnson EF, Marques-Soares C, Dansette PM, Mansuy D, Stout CD (2003) Structure of a substrate complex of mammalian cytochrome P450 2C5 at 2.3 Å resolution: evidence for multiple substrate binding modes. Biochemistry 42:6370–6379
Williams PA, Cosme J, Ward A, Angove HC, Vinkovic DM, Jhoti H (2003) Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature 424:464–468
Wester MR, Yano JK, Schoch GA, Yang C, Griffin KJ, Stout CD, Johnson EF (2004) The structure of human cytochrome P 450 2C9 complexed with flurbiprofen at 2.0-Å resolution. J Biol Chem 279:35630–35637
Scott EE, He YA, Wester MR, White MA, Chin CC, Halpert JR, Johnson EF, Stout CD (2003) An open conformation of mammalian cytochrome P 450 2B4 at 1.6-Å resolution. Proc Natl Acad Sci USA 100:13196–13201
Rowland P, Blaney Frank E, Smyth Martin G, Jones Jo J, Leydon Vaughan R, Oxbrow Amanda K, Lewis Ceri J, Tennant Mike G, Modi S, Eggleston Drake S, Chenery Richard J, Bridges Angela M (2006) Crystal structure of human cytochrome P450 2D6. J Biol Chem 281:7614–7622
de Graaf C, Oostenbrink C, Keizers PHJ, van der Wijst T, Jongejan A, Vermeulen NPE (2006) Catalytic site prediction and virtual screening of cytochrome P450 2D6 substrates by consideration of water and rescoring in automated docking. J Med Chem 49:2417–2430
Schrodinger, Glide version 4.5, LLC, Portland, OR, USA, 2007
de Graaf C, Vermeulen NPE, Feenstra KA (2005) Cytochrome P450 in silico: an integrative modeling approach. J Med Chem 48:2725–2755
Lewis David FV, Dickins M, Lake Brian G, Goldfarb Peter S (2003) A molecular model of CYP2D6 constructed by homology with the CYP2C5 crystallographic template: investigation of enzyme–substrate interactions. Drug Metabol Drug Interact 19:189–210
Venhorst J, ter Laak AM, Commandeur JNM, Funae Y, Hiroi T, Vermeulen NPE (2003) Homology modeling of rat and human cytochrome P450 2D (CYP2D) isoforms and computational rationalization of experimental ligand-binding specificities. J Med Chem 46:74–86
de Graaf C, Oostenbrink C, Keizers PHJ, van Vugt-Lussenburg BMA, van Waterschoot RAB, Tschirret-Guth RA, Commandeur JNM, Vermeulen NPE (2007) Molecular modeling-guided site-directed mutagenesis of cytochrome P450 2D6. Curr Drug Metab 8:59–77
Sali A, Blundell TL (1993) Comparative protein modeling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Shen M-Y, Sali A (2006) Statistical potential for assessment and prediction of protein structures. Protein Sci 15:2507–2524
Davis IW, Murray LW, Richardson JS, Richardson DC (2004) MolPROBITY: structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res 32:W615–W619
Modi S, Paine MJ, Sutcliffe MJ, Lian LY, Primrose WU, Wolf CR, Roberts GC (1996) A model for human cytochrome P450 2D6 based on homology modeling and NMR studies of substrate binding. Biochemistry 35:4540–4550
Schrodinger, Maestro version 7.5.116, LLC, Portland, OR, USA, 2006
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 118:11225–11236
Halgren TA (1996) Merck molecular force field. V. Extension of MMFF94 using experimental data, additional computational data, and empirical rules. J Comput Chem 17:616–641
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47:1750–1759
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J Med Chem 49:6177–6196
Flanagan JU, Marechal J-D, Ward R, Kemp CA, McLaughlin LA, Sutcliffe MJ, Roberts GCK, Paine MJI, Wolf CR (2004) Phe120 contributes to the regiospecificity of cytochrome P450 2D6: mutation leads to the formation of a novel dextromethorphan metabolite. Biochem J 380:353–360
Paine MJI, McLaughlin LA, Flanagan JU, Kemp CA, Sutcliffe MJ, Roberts GCK, Wolf CR (2003) Residues glutamate 216 and aspartate 301 are key determinants of substrate specificity and product regioselectivity in cytochrome P450 2D6. J Biol Chem 278:4021–4027
Gotoh O (1992) Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J Biol Chem 267:83–90
McLaughlin LA, Paine MJI, Kemp CA, Marechal J-D, Flanagan JU, Ward CJ, Sutcliffe MJ, Roberts GCK, Wolf CR (2005) Why is quinidine an inhibitor of cytochrome P450 2D6?: the role of key active-site residues in quinidine binding. J Biol Chem 280:38617–38624
Ellis SW, Hayhurst GP, Lightfoot T, Smith G, Harlow J, Rowland-Yeo K, Larsson C, Mahling J, Lim CK, Wolf CR, Blackburn MG, Lennard MS, Tucker GT (2000) Evidence that serine 304 is not a key ligand-binding residue in the active site of cytochrome P450 2D6. Biochem J 345:565–571
Ellis SW, Rowland K, Ackland MJ, Rekka E, Simula AP, Lennard MS, Wolf CR, Tucker GT (1996) Influence of amino acid residue 374 of cytochrome P-450 2D6 (CYP2D6) on the regio- and enantio-selective metabolism of metoprolol. Biochem J 316:647–654
Hayhurst GP, Harlow J, Chowdry J, Gross E, Hilton E, Lennard MS, Tucker GT, Ellis SW (2001) Influence of phenylalanine-481 substitutions on the catalytic activity of cytochrome P450 2D6. Biochem J 355:373–379
Ellis SW, Hayhurst GP, Smith G, Lightfoot T, Wong MMS, Simula AP, Ackland MJ, Sternberg MJE, Lennard MS et al (1995) Evidence that aspartic acid 301 is a critical substrate-contact residue in the active site of cytochrome P450 2D6. J Biol Chem 270:29055–29058
Kirton SB, Kemp CA, Tomkinson NP, St.-Gallay S, Sutcliffe MJ (2002) Impact of incorporating the 2C5 crystal structure into comparative models of cytochrome P450 2D6. Proteins Struct Funct Genet 49:216–231
Guengerich FP, Hanna IH, Martin MV, Gillam EMJ (2003) Role of glutamic acid 216 in cytochrome P450 2D6 substrate binding and catalysis. Biochemistry 42:1245–1253
Keizers PHJ, Lussenburg BMA, de Graaf C, Mentink LM, Vermeulen NPE, Commandeur JNM (2004) Influence of phenylalanine 120 on cytochrome P450 2D6 catalytic selectivity and regiospecificity: crucial role in 7-methoxy-4-(aminomethyl)-coumarin metabolism. Biochem Pharmacol 68:2263–2271
Imai T, Taketani M, Suzu T, Kusube K, Otagiri M (1999) In vitro identification of the human cytochrome P-450 enzymes involved in the N-demethylation of azelastine. Drug Metab Dispos 27:942–946
Nakajima M, Nakamura S, Tokudome S, Shimada N, Yamazaki H, Yokoi T (1999) Azelastine N-demethylation by cytochrome P-450 (CYP)3A4, CYP2D6, and CYP1A2 in human liver microsomes: evaluation of approach to predict the contribution of multiple CYPs. Drug Metab Dispos 27:1381–1391
Johnston GD, Finch MB, Shanks RG (1986) Peripheral vascular effects of bufuralol in hypertensive and normal subjects: a comparison with propranolol and pindolol. Eur J Clin Pharmacol 30:649–652
Masuda K, Tamagake K, Okuda Y, Torigoe F, Tsuzuki D, Isobe T, Hichiya H, Hanioka N, Yamamoto S, Narimatsu S (2005) Change in enantioselectivity in bufuralol 1″-hydroxylation by the substitution of phenylalanine-120 by alanine in cytochrome P450 2D6. Chirality 17:37–43
Kerry NL, Somogyi AA, Bochner F, Mikus G (1994) The role of CYP2D6 in primary and secondary oxidative metabolism of dextromethorphan: in vitro studies using human liver microsomes. Br J Clin Pharmacol 38:243–248
Yu A, Dong H, Lang D, Haining RL (2001) Characterization of dextromethorphan O- and N-demethylation catalyzed by highly purified recombinant human CYP2D6. Drug Metab Dispos 29:1362–1365
Margolis JM, O’Donnell JP, Mankowski DC, Ekins S, Obach RS (2000) (R)-, (S)-, and racemic fluoxetine N-demethylation by human cytochrome P450 enzymes. Drug Metab Dispos 28:1187–1191
Rao S, Sanschagrin PC, Greenwood JR, Repasky MP, Sherman W, Farid R (2008) Improving database enrichment through ensemble docking. J Comput Aided Mol Des 22:621–627
Sherman W, Day T, Jacobson MP, Friesner RA, Farid R (2006) Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem 49:534–553
Hritz J, de Ruiter A, Oostenbrink C (2008) Impact of plasticity and flexibility on docking results for cytochrome P450 2D6: a combined approach of molecular dynamics and ligand docking. J Med Chem 51:7469–7477
Kudo S, Uchida M, Odomi M (1997) Metabolism of carteolol by cDNA-expressed human cytochrome P450. Eur J Clin Pharmacol 52:479–485
Dayer P, Desmeules J, Leemann T, Striberni R (1988) Bioactivation of the narcotic drug codeine in human liver is mediated by the polymorphic monooxygenase catalyzing debrisoquine 4-hydroxylation (cytochrome P-450 dbl/bufI). Biochem Biophys Res Commun 152:411–416
Eiermann B, Edlund PO, Tjernberg A, Dalen P, Dahl M-L, Bertilsson L (1998) 1- and 3-Hydroxylations, in addition to 4-hydroxylation, of debrisoquine are catalyzed by cytochrome P450 2D6 in humans. Drug Metab Dispos 26:1096–1101
Lightfoot T, Ellis SW, Mahling J, Ackland MJ, Blaney FE, Bijloo GJ, De Groot MJ, Vermeulen NPE, Blackburn GM, Lennard MS, Tucker GT (2000) Regioselective hydroxylation of debrisoquine by cytochrome P4502D6: implications for active site modelling. Xenobiotica 30:219–233
Kalant H (2001) The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. Can Med Assoc J 165:917–928
Meyer MR, Peters FT, Maurer HH (2008) The role of human hepatic cytochrome P450 isozymes in the metabolism of racemic 3,4-methylenedioxymethamphetamine and its enantiomers. Drug Metab Dispos 36:2345–2354
Geertsen S, Foster BC, Wilson DL, Cyr TD, Casley W (1995) Metabolism of methoxyphenamine and 2-methoxyamphetamine in P4502D6-transfected cells and cell preparations. Xenobiotica 25:895–906
Bach MV, Coutts RT, Baker GB (1999) Involvement of CYP2D6 in the in vitro metabolism of amphetamine, two N-alkylamphetamines and their 4-methoxylated derivatives. Xenobiotica 29:719–732
Abraham WT (2000) Beta-blockers: the new standard of therapy for mild heart failure. Arch Intern Med 160:1237–1247
Singh BN, Williams EMV (1972) Mode of action of a new antidysrhythmic drug, Ko 1173. Br J Pharmacol 44:1–9
Dejgard A, Petersen P, Kastrup J (1988) Mexiletine for treatment of chronic painful diabetic neuropathy. Lancet 1:9–11
Knoche B, Gehrcke B, Koenig A, Wainer IW (1996) Determination of the enantiomeric composition of mexiletine and its four hydroxylated metabolites in urine by enantioselective capillary gas chromatography. Chirality 8:30–34
Chow T, Hiroi T, Imaoka S, Chiba K, Funae Y (1999) Isoform-selective metabolism of mianserin by cytochrome P-450 2D. Drug Metab Dispos 27:1200–1204
Postlind H, Danielson A, Lindgren A, Andersson SHG (1998) Tolterodine, a new muscarinic receptor antagonist, is metabolized by cytochromes P450 2D6 and 3A in human liver microsomes. Drug Metab Dispos 26:289–293
Brynne N, Svanstrom C, Aberg-Wistedt A, Hallen B, Bertilsson L (1999) Fluoxetine inhibits the metabolism of tolterodine-pharmacokinetic implications and proposed clinical relevance. Br J Clin Pharmacol 48:553–563
Eap CB, Lessard E, Baumann P, Brawand-Amey M, Yessine M-A, O’Hara G, Turgeon J (2003) Role of CYP2D6 in the stereoselective disposition of venlafaxine in humans. Pharmacogenetics 13:39–47
Acknowledgments
The authors thank Yuchen Bai from the Wyeth Bioinformatics Department for his help with sequence analysis of CYP2D6 polymorphs and Will Somers for support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rayomand J. Unwalla and Jason B. Cross contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Unwalla, R.J., Cross, J.B., Salaniwal, S. et al. Using a homology model of cytochrome P450 2D6 to predict substrate site of metabolism. J Comput Aided Mol Des 24, 237–256 (2010). https://doi.org/10.1007/s10822-010-9336-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-010-9336-6