Abstract
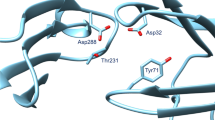
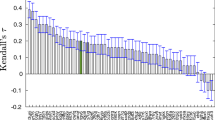
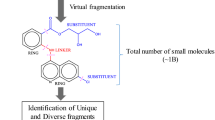
The ability to identify fragments that interact with a biological target is a key step in FBDD. To date, the concept of fragment based drug design (FBDD) is increasingly driven by bio-physical methods. To expand the boundaries of QSAR paradigm, and to rationalize FBDD using In silico approach, we propose a fragment based QSAR methodology referred here in as FB-QSAR. The FB-QSAR methodology was validated on a dataset consisting of 52 Hydroxy ethylamine (HEA) inhibitors, disclosed by GlaxoSmithKline Pharmaceuticals as potential anti-Alzheimer agents. To address the issue of target selectivity, a major confounding factor in the development of selective BACE1 inhibitors, FB-QSSR models were developed using the reported off target activity values. A heat map constructed, based on the activity and selectivity profile of the individual R-group fragments, and was in turn used to identify superior R-group fragments. Further, simultaneous optimization of multiple properties, an issue encountered in real-world drug discovery scenario, and often overlooked in QSAR approaches, was addressed using a Multi Objective (MO-QSPR) method that balances properties, based on the defined objectives. MO-QSPR was implemented using Derringer and Suich desirability algorithm to identify the optimal level of independent variables (X) that could confer a trade-off between selectivity and activity. The results obtained from FB-QSAR were further substantiated using MIF (Molecular Interaction Fields) studies. To exemplify the potentials of FB-QSAR and MO-QSPR in a pragmatic fashion, the insights gleaned from the MO-QSPR study was reverse engineered using Inverse-QSAR in a combinatorial fashion to enumerate some prospective novel, potent and selective BACE1 inhibitors.





Similar content being viewed by others
References
Hansch C, Fujita T (1964) ρ-σ-π analysis. A method for the correlation of biological activity and chemical structure. J Am Chem Soc 86:1616–1626
Zartler ER, Shapiro MJ (2005) Fragonomics: Fragment-based drug discovery. Curr Opin Chem Biol 9:366–370
Verdonk ML, Hartshorn MJ (2004) Structure-guided fragment screening for lead discovery. Curr Opin Drug Discov Dev 7:404–410
Miranker A, Karplus M (1991) Functionality maps of binding sites: a multiple copy simultaneous search method. Proteins 11:29–34
Caflisch A, Miranker A, Karplus M (1993) Multiple copy simultaneous search and construction of ligands in binding sites. J Med Chem 36:2142–2167
Evensen E, Joseph-McCarthy D, Karplus M (1997) MCSSV2. Harvard University, Cambridge
Goodford PJ (1985) A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J Med Chem 28:849–857
Jencks WP (1981) On the attribution and additivity of binding energies. Proc Natl Acad Sci USA 78:4046–4050
Free SM, Wilson JW (1964) A mathematical contribution to structure- activity studies. J Med Chem 7:395–399
Fujita T, Ban T (1971) Structure-activity study of phenethylamines as substrates of biosynthetic enzymes of sympathetic transmitters. J Med Chem 14:148–152
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M (1999) β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–741
Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME (1999) Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature 402:533–537
Selkoe DJ (1999) Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature 399:A23–A31
Baldwin ET, Bhat TN, Gulnik S, Hosur MV, Sowder RC 2nd, Cachau RE, Collins J, Silva AM, Erickson JW (1993) Crystal structures of native and inhibited forms of human cathepsin D: implications for lysosomal targeting and drug design. Proc Natl Acad Sci USA 90:6796–6800
Saunders AJ, Kim TW, Tanzi RE (1999) BACE maps to chromosome 11 and a BACE homolog, BACE2, reside in the obligate down syndrome region of chromosome 21. Science 286:1255a
Charrier N, Clarke B, Cutler L, Demont E, Dingwall C, Dunsdon R, East P, Hawkins J, Howes C, Hussain I, Jeffrey P, Maile G, Matico R, Mosley J, Naylor A, O’Brien A, Redshaw S, Rowland P, Soleil V, Smith JK, Sweitzer S, Theobald P, Vesey D, Walter SD, Wayne G (2008) Second generation of hydroxyethylamine BACE-1 inhibitors: optimizing potency and oral bioavailability. J Med Chem 51:3313–3317
Clarke B, Demont E, Dingwall C, Dunsdon R, Faller A, Hawkins J, Hussain I, MacPherson D, Maile G, Matico R, Milner P, Mosley J, Naylor AA, O’Brien A, Redshaw S, Riddell D, Rowland P, Soleil V, Smith JK, Stanway S, Stemp G, Sweitzer S, Theobald P, Vesey D, Walter DS, Ward J, Wayne G (2008) BACE-1 inhibitors part 1: identification of novel hydroxy ethylamines (HEAs). Bioorg Med Chem Lett 18:1011–1016
Clarke B, Demont E, Dingwall C, Dunsdon R, Faller A, Hawkins J, Hussain I, MacPherson D, Maile G, Matico R, Milner P, Mosley J, Naylor AA, O’Brien A, Redshaw S, Riddell D, Rowland P, Soleil V, Smith JK, Stanway S, Stemp G, Sweitzer S, Theobald P, Vesey D, Walter DS, Ward J, Wayne G (2008) BACE-1 inhibitors part 2: Identification of hydroxyl ethylamines (HEAs) with reduced peptidic character. Bioorg Med Chem Lett 18:1017–1021
Beswick P, Charrier N, Clarke B, Demont E, Dingwall C, Dunsdon R, Faller A, Gleave R, Hawkins J, Hussain I, Johnson CN, MacPherson D, Maile G, Milner P, Naylor A, O’Brien A, Redshaw S, Riddell D, Rowland P, Skidmore J, Soleil V, Smith K, Stanway S, Stemp G, Stuart A, Theobald P, Vesey D, Walter DS, Ward J, Wayne G (2008) BACE-1 inhibitors. Part 3: identification of hydroxyl ethylamines (HEAs) with nanomolar potency in cells. Bioorg Med Chem Lett 18:1022–1026
Congreve M, Aharony D, Albert J, Callaghan O, Campbell J, Carr RA, Chessari G, Cowan S, Edwards PD, Frederickson M, McMenamin R, Murray CW, Patel S, Wallis N (2007) Application of fragment screening by X-ray crystallography to the discovery of aminopyridines as inhibitors of beta-secretase. J Med Chem 50:1124–1132
Murray CW, Callaghan O, Chessari G, Cleasby A, Congreve M, Frederickson M, Hartshorn JM, McMenamin R, Patel S, Wallis N (2007) Application of fragment screening by X-ray crystallography to the discovery of aminopyridines as inhibitors of beta-secretase. J Med Chem 50:1116–1123
Nicolaou AC, Brown N, Pattichis SC (2007) Molecular optimization using computational multi-objective methods. Curr Opin Drug Discov Devel 3:316–324
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Quality Technol 12:214–219
Hann MM, Leach AR, Harper G (2001) Molecular complexity and its impact on the probability of finding leads for drug discovery. J Chem Inf Comput Sci 41:856–864
HyperChem 7.52 Hypercube Inc., Gainesville Fl 32608 USA
Molecular Operating EnVironment (MOE) (2009) Chemical computing group, Montreal
SPSS Version 15.0 (2009) SPSS Inc., Chicago
Vijayan RS, Ghoshal N (2008) Structural basis for ligand recognition at the benzodiazepine binding site of GABAA alpha 3 receptor, and pharmacophore-based virtual screening approach. J Mol Graph Model 27:286–298
TSAR Version 3.3 (2007) Accelrys Inc., San Diego
Hajduk PJ (2006) Puzzling through fragment-based drug design. Nat Chem Biol 12:658–659
Cruz-Monteagudo M, Borges F, Cordeiro MN (2008) Desirability-based multiobjective optimization for global QSAR studies: application to the design of novel NSAIDs with improved analgesic, antiinflammatory, and ulcerogenic profiles. J Comput Chem 29:2445–2459
STATISTICA, 6.0 (2001) Statsoft_Inc
Laskowski RA, Thornton JM, Humblet C, Singh J (1996) X-SITE: use of empirically derived atomic packing preferences to identify favourable interaction regions in the binding sites of proteins. J Mol Biol 259:175–201
Nissink JWM, Verdonk ML, Klebe G (2000) Simple knowledge-based descriptors to predict protein-ligand interactions. methodology and validation. J Comput Aided Mol Des 14:787–803
Bissantz C, Kuhn B, Stahl M (2010) A medicinal chemist’s guide to molecular interactions. J Med Chem 53:5061–5084
Panigrahi SK, Desiraju GR (2007) Strong and weak hydrogen bonds in the protein-ligand interface. Proteins 67:128–141
Desiraju GR, Sarkel S (2004) N–H …O, O–H …O, and C–H …O hydrogen bonds in protein-ligand complexes: strong and weak interactions in molecular recognition. Proteins 54:247–259
Barratt E, Bronowska A, Vondrasek J, Cerny J, Bingham R, Phillips S, Homans SW (2006) Thermodynamic penalty arising from burial of a ligand polar group within a hydrophobic pocket of a protein receptor. J Mol Biol 362:994–1003
Verloop A, Hoogenstraaten W, Tipker J (1976) Development and application of new steric substituent parameters in drug design. In Drug Design Academic Press, New York
Hall LH, Kier LB (1992) The molecular connectivity chi indexes and kappa shape indexes in structure-property modeling, Reviews in Computational Chemistry ed, Wiley
Hansch C, Leo AJ (1979) Substituent constants for correlation analysis in chemistry and biology. Wiley, New York
Golbraikh A, Tropsha A (2002) Beware of q2. J Mol Graph Model 20:269–276
Cronin MTD, Schultz TW (2003) Pitfalls in QSARs. THEOCHEM 622:39–52
Eriksson L, Jaworska J, Worth AP, Cronin MT, McDowell RM, Gramatica P (2003) Methods for reliability and uncertainty assessment and for applicability evaluations of classification and regression based QSARs. Environ Health Perspect 111:1361–1375
Pavlidis P, Noble WS (2003) Matrix2png: a utility for visualizing matrix data. Bioinformatics 19:295–296
OpenEye (2006) OpenEye Scientific Software Santa Fe NM
Acknowledgments
The authors thank Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing a financial grant under the Mission Mode Program CMM 0017. M.P thanks CSIR for a Project Fellowship and R.S.K.V thanks CSIR for a Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manoharan, P., Vijayan, R.S.K. & Ghoshal, N. Rationalizing fragment based drug discovery for BACE1: insights from FB-QSAR, FB-QSSR, multi objective (MO-QSPR) and MIF studies. J Comput Aided Mol Des 24, 843–864 (2010). https://doi.org/10.1007/s10822-010-9378-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-010-9378-9