Abstract
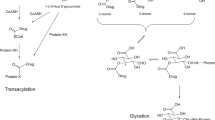
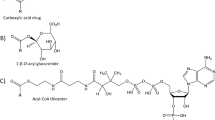
Drugs and drug candidates containing a carboxylic acid moiety, including many widely used non-steroidal anti-inflammatory drugs (NSAIDs) are often metabolized to form acyl glucuronides (AGs). NSAIDs such as Ibuprofen are amongst the most widely used drugs on the market, whereas similar carboxylic acid drugs such as Suprofen have been withdrawn due to adverse events. Although the link between these AG metabolites and toxicity is not proven, there is circumstantial literature evidence to suggest that more reactive acyl glucuronides may, in some cases, present a greater risk of exhibiting toxic effects. We wished therefore to rank the reactivity of potential new carboxylate-containing drug candidates, and performed kinetic studies on synthetic acyl glucuronides to benchmark our key compounds. Driven by the desire to quickly rank the reactivity of compounds without the need for lengthy synthesis of the acyl glucuronide, a correlation was established between the degradation half-life of the acyl glucuronide and the half life for the hydrolysis of the more readily available methyl ester derivative. This finding enabled a considerable broadening of chemical property space to be investigated. The need for kinetic measurements was subsequently eliminated altogether by correlating the methyl ester hydrolysis half-life with the predicted 13C NMR chemical shift of the carbonyl carbon together with readily available steric descriptors in a PLS model. This completely in silico prediction of acyl glucuronide reactivity is applicable within the earliest stages of drug design with low cost and acceptable accuracy to guide intelligent molecular design. This reactivity data will be useful alongside the more complex additional pharmacokinetic exposure and distribution data that is generated later in the drug discovery process for assessing the overall toxicological risk of acidic drugs.








Similar content being viewed by others
References
Fung M, Thornton A, Mybeck K, Hornbuckle K, Muniz E (2001) Drug Inf J 35:293
Faed EM (1984) Drug Metab Dispos 15:1213
Regan SL, Maggs JL, Hammond TG, Lambert C, Williams DP, Park BK (2010) Biopharm Drug Dispos 31:367
Baillie TA (2008) Chem Res Toxicol 21:129
Guengerich F, MacDonald J (2007) Chem Res Toxicol 20:344
Walgren J, Mitchell M, Thompson D (2005) Crit Rev Toxicol 35:325
Koga T, Fujiwara R, Nakajima M, Yokoi T (2011) Drug Metab Dispos 39:54
Sawamura R, Okudaira N, Watanabe K, Murai T, Kobayashi Y, Tachibana M, Ohnuki T, Masuda K, Honma H, Kurihara A, Okazaki O (2010) Drug Metab Dispos 38:1857
Benet L, Spahn-Langguth H, Iwakawa S, Volland C, Mizuma T, Mayer S, Mutschler E, Lin E (1993) Life Sci 53:141
Bolze S, Bromet N, Gay-Feutry C, Massiere F, Boulieu R, Hulot T (2002) Drug Metab Dispos 30:404
Vanderhoeven S, Troke J, Tranter G, Wilson I, Nicholson J, Lindon J (2004) Xenobiotica 34:889
Yoshioka T, Baba A (2009) Chem Res Toxicol 22:1559
Bowkett ER, Harding JA, Maggs JL, Park BK, Perriea JA, Stachulski AV (2007) Tetrahedron 63:7596
Wold S, Geladi P, Esbensen K, Öhman J (1987) J Chemom 1:41
Verloop A (1987) The STERIMOL approach to drug design. Dekker, New York
Neuvonen H, Neuvonen K (1999) J Chem Soc Perkin Trans 2:1497
Fröhlich J, Berger S (2008) Eur J Org Chem 9:1632
Chimichi S, Boccalini M, Matteucci A, Kharlamov SV, Latypov SK, Sinyashin OG (2010) Magn Reson Chem 48:607
Smith SG, Goodman JM (2010) J Am Chem Soc 132:12946
Sarotti AM, Pellegrinet SC (2009) J Org Chem 74:7254
Jain R, Bally T, Rablen PR (2009) J Org Chem 74:4017
Stachulsky A, Harding J, Lindon J, Maggs J, Park K, Wilson I (2006) J Med Chem 49:6931
Hasegawa H, Akira K, Shinohara Y, Kasuya Y, Hashimoto T (2001) Biol Pharm Bull 24:852
Baba A, Yoshioka T (2009) Chem Res Toxicol 22:1998
Acknowledgments
We thank Natalie de Sousa for HPLC purification of the biosynthetic AGs.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Potter, T., Lewis, R., Luker, T. et al. In silico prediction of acyl glucuronide reactivity. J Comput Aided Mol Des 25, 997–1005 (2011). https://doi.org/10.1007/s10822-011-9479-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-011-9479-0