Abstract
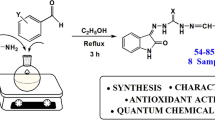
Phenolic Schiff bases are known for their diverse biological activities and ability to scavenge free radicals. To elucidate (1) the structure–antioxidant activity relationship of a series of thirty synthetic derivatives of 2-methoxybezohydrazide phenolic Schiff bases and (2) to determine the major mechanism involved in free radical scavenging, we used density functional theory calculations (B3P86/6-31+(d,p)) within polarizable continuum model. The results showed the importance of the bond dissociation enthalpies (BDEs) related to the first and second (BDEd) hydrogen atom transfer (intrinsic parameters) for rationalizing the antioxidant activity. In addition to the number of OH groups, the presence of a bromine substituent plays an interesting role in modulating the antioxidant activity. Theoretical thermodynamic and kinetic studies demonstrated that the free radical scavenging by these Schiff bases mainly proceeds through proton-coupled electron transfer rather than sequential proton loss electron transfer, the latter mechanism being only feasible at relatively high pH.





Similar content being viewed by others
References
Rice-Evans C, Miller NJ, Paganga G (1996) Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Halliwell B, Aeschbach R, Löliger J, Aruoma OI (1995) The characterization of antioxidants. Food Chem Toxicol 33:601–617
Anouar E, Calliste C, Kosinova P, Di Meo F, Duroux J, Champavier Y, Marakchi K, Trouillas P (2009) Free radical scavenging properties of guaiacol oligomers: a combined experimental and quantum study of the guaiacyl-moiety role. J Phys Chem A 113:13881–13891
Vicini P, Geronikaki A, Incerti M, Busonera B, Poni G, Cabras CA, La Colla P (2003) Synthesis and biological evaluation of benzo [d] isothiazole, benzothiazole and thiazole Schiff bases. Bioorg Med Chem 11:4785–4789
Tarafder M, Kasbollah A, Saravanan N, Crouse KA, Ali AM (2002) S-methyldithiocarbazate and its Schiff bases: evaluation of bondings and biological properties. J Biochem Mol Biol Biophys 6:85–91
Khan KM, Ahmad A, Ambreen N, Amyn A, Perveen S, Khan SA, Choudhary MI (2009) Schiff bases of 3-formylchromones as antibacterial, antifungal, and phytotoxic agents. Lett Drug Des Discov 6:363–373
Chohan ZH, Pervez H, Rauf A, Khan KM, Supuran CT (2006) Antibacterial cobalt (II), copper (II), nickel (II) and zinc (II) complexes of mercaptothiadiazole-derived furanyl, thienyl, pyrrolyl, salicylyl and pyridinyl Schiff bases. J Enzyme Inhib Med Chem 21:193–201
Chohan ZH, Pervez H, Rauf A, Khan KM, Maharvi GM, Supuran CT (2004) Antibacterial and antifungal mono-and di-substituted symmetrical and unsymmetrical triazine-derived Schiff-bases and their transition metal complexes. J Enzyme Inhib Med Chem 19:161–168
Kabeer AS, Baseer M, Mote N (2001) Synthesis and antimicrobial activity of some Schiff bases from benzothiazoles. Asian J Chem 13:496–500
Chohan ZH, Arif M, Shafiq Z, Yaqub M, Supuran CT (2006) In vitro antibacterial, antifungal & cytotoxic activity of some isonicotinoylhydrazide Schiff’s bases and their cobalt (II), copper (II), nickel (II) and zinc (II) complexes. J Enzyme Inhib Med Chem 21:95–103
Guo Z, Xing R, Liu S, Zhong Z, Ji X, Wang L, Li P (2007) Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr Res 342:1329–1332
Taha M, Baharudin MS, Ismail NH, Khan KM, Jaafar FM, Siddiqui S, Iqbal Choudhary M (2013) Synthesis of 2-methoxybenzoylhydrazone and evaluation of their antileishmanial activity. Bioorg Med Chem Lett 23:3463–3466
Mohammed Khan K, Taha M, Naz F, Siddiqui S, Ali S, Rahim F, Perveen S, Iqbal Choudhary M (2012) Acylhydrazide Schiff bases: DPPH radical and superoxide anion scavengers. Med Chem 8:705–710
Mohammed Khan K, Shah Z, Uddin Ahmad V, Khan M, Taha M, Rahim F, Ali S, Ambreen N, Perveen S, Iqbal Choudhary M (2012) 2,4,6-Trichlorophenylhydrazine Schiff bases as DPPH radical and super oxide anion scavengers. Med Chem 8:452–461
Trouillas P, Marsal P, Siri D, Lazzaroni R, Duroux J-L (2006) A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: the specificity of the 3-OH site. Food Chem 97:679–688
Kozlowski D, Trouillas P, Calliste C, Marsal P, Lazzaroni R, Duroux J-L (2007) Density functional theory study of the conformational, electronic, and antioxidant properties of natural chalcones. J Phys Chem A 111:1138–1145
Leopoldini M, Russo N, Toscano M (2011) The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem 125:288–306
Wang L-F, Zhang H-Y (2003) A theoretical investigation on DPPH radical-scavenging mechanism of edaravone. Bioorg Med Chem Lett 13:3789–3792
Netto LE, Chae HZ, Kang S-W, Rhee SG, Stadtman ER (1996) Removal of hydrogen peroxide by thiol-specific antioxidant enzyme (TSA) is involved with its antioxidant properties TSA possesses thiol peroxidase activity. J Biol Chem 271:15315–15321
Halliwell B (1991) Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med 91:S14–S22
Di Mascio P, Murphy ME, Sies H (1991) Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr 53:194S–200S
Saito H, Ishihara K (1997) Antioxidant activity and active sites of phospholipids as antioxidants. J Am Oil Chem Soc 74:1531–1536
Padmaja A, Rajasekhar C, Muralikrishna A, Padmavathi V (2011) Synthesis and antioxidant activity of oxazolyl/thiazolylsulfonylmethyl pyrazoles and isoxazoles. Eur J Med Chem 46:5034–5038
Brede O, Ganapathi MR, Naumov S, Naumann W, Hermann R (2001) Localized electron transfer in nonpolar solution: reaction of phenols and thiophenols with free solvent radical cations. J Phys Chem A 105:3757–3764
DiLabio GA, Johnson ER (2007) Lone pair-π and π–π interactions play an important role in proton-coupled electron transfer reactions. J Am Chem Soc 129:6199–6203
Hatcher E, Soudackov AV, Hammes-Schiffer S (2007) Proton-coupled electron transfer in soybean lipoxygenase: dynamical behavior and temperature dependence of kinetic isotope effects. J Am Chem Soc 129:187–196
Hammes-Schiffer S (2012) Proton-coupled electron transfer: classification scheme and guide to theoretical methods. Energy Environ Sci 5:7696–7703
Lingwood M, Hammond JR, Hrovat DA, Mayer JM, Borden WT (2006) MPW1 K performs much better than B3LYP in DFT calculations on reactions that proceed by proton-coupled electron transfer (PCET). J Chem Theory Comput 2:740–745
Di Meo F, Lemaur V, Cornil J, Lazzaroni R, Duroux J-L, Olivier Y, Trouillas P (2013) Free radical scavenging by natural polyphenols: atom versus electron transfer. J Phys Chem A 117:2082–2092
Musialik M, Kuzmicz R, Pawlowski Tomasz S, Litwinienko G (2009) Acidity of hydroxyl groups: an overlooked influence on antiradical properties of flavonoids. J Org Chem 74:2699–2709
Trouillas P, Marsal P, Svobodova A, Vostalova J, Gazak R, Hrbac J, Sedmera P, Kren V, Lazzaroni R, Duroux J-L, Walterova D (2008) Mechanism of the antioxidant action of silybin and 2,3-dehydrosilybin flavonolignans: a joint experimental and theoretical study. J Phys Chem A 112:1054–1063
Iuga C, Alvarez-Idaboy JRl, Russo N (2012) Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: a quantum chemical and computational kinetics study. J Org Chem 77:3868–3877
Leopoldini M, Chiodo SG, Russo N, Toscano M (2011) Detailed investigation of the OH radical quenching by natural antioxidant caffeic acid studied by quantum mechanical models. J Chem Theory Comput 7:4218–4233
Alberto ME, Russo N, Grand A, Galano A (2013) A physicochemical examination of the free radical scavenging activity of Trolox: mechanism, kinetics and influence of the environment. Phys Chem Chem Phys 15:4642–4650
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Frisch MJ et al (2009) Gaussian 09, Revision A.02. Gaussian, Inc., Wallingford, CT
Anouar EH, Gierschner J, Duroux J-L, Trouillas P (2012) UV/Visible spectra of natural polyphenols: a time-dependent density functional theory study. Food Chem 131:79–89
Košinová P, Di Meo F, Anouar EH, Duroux JL, Trouillas P (2011) H-atom acceptor capacity of free radicals used in antioxidant measurements. Int J Quantum Chem 111:1131–1142
Chiodo SG, Leopoldini M, Russo N, Toscano M (2010) The inactivation of lipid peroxide radical by quercetin. A theoretical insight. Phys Chem Chem Phys 12:7662–7670
Zhao Y, Truhlar DG (2004) Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: the MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals interactions. J Phys Chem A 108:6908–6918
Tejero I, González-García N, González-Lafont À, Lluch JM (2007) Tunneling in green tea: understanding the antioxidant activity of catechol-containing compounds. A variational transition-state theory study. J Am Chem Soc 129:5846–5854
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093
Guerra M, Amorati R, Pedulli GF (2004) Water effect on the OH dissociation enthalpy of para-substituted phenols: a DFT study. J Org Chem 69:5460–5467
Farazdel A, Dupuis M, Clementi E, Aviram A (1990) Electric-field induced intramolecular electron transfer in spiro.pi.-electron systems and their suitability as molecular electronic devices. A theoretical study. J Am Chem Soc 112:4206–4214
Grimme S (2004) Accurate description of van der Waals complexes by density functional theory including empirical corrections. J Comput Chem 25:1463–1473
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104–154123
Di Meo F, Sancho Garcia JC, Dangles O, Trouillas P (2012) Highlights on anthocyanin pigmentation and copigmentation: a matter of flavonoid π-stacking complexation to be described by DFT-D. J Chem Theory Comput 8:2034–2043
Neese F (2012) The ORCA program system. Wiley Interdiscip Rev Comput Mol Sci 2:73–78
Valiev M, Bylaska EJ, Govind N, Kowalski K, Straatsma TP, Van Dam HJJ, Wang D, Nieplocha J, Apra E, Windus TL, de Jong WA (2010) NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations. Comput Phys Commun 181:1477–1489
Neese F, Wennmohs F, Hansen A, Becker U (2009) Efficient, approximate and parallel Hartree-Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree-Fock exchange. Chem Phys 356:98–109
Mahoney LR, Mendenhall GD, Ingold KU (1973) Calorimetric and equilibrium studies on some stable nitroxide and iminoxy radicals. Approximate oxygen-hydrogen bond dissociation energies in hydroxylamines and oximes. J Am Chem Soc 95:8610–8614
Acknowledgments
The authors thank the “Conseil Régional du Limousin” for CALI (CAlcul en LImousin) and Atta-ur-Rahman Institute for Natural Product Discovery, Universiti Teknologi for computing facilities. The authors thank the “Pharmacology and Toxicology Research Laboratory-Faculty of Pharmacy, Universiti Teknologi MARA, Puncak Alam Campus, Malaysia” for antioxidant facilities. P.T. gratefully acknowledges the support by the Operational Program Research and Development for Innovations–European Regional Development Fund (project CZ.1.05/2.1.00/03.0058 of the Ministry of Education, Youth and Sports of the Czech Republic). I.B. and P.T. gratefully thank the ‘Association Djerbienne de France’ (ADF) for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anouar, E.H., Raweh, S., Bayach, I. et al. Antioxidant properties of phenolic Schiff bases: structure–activity relationship and mechanism of action. J Comput Aided Mol Des 27, 951–964 (2013). https://doi.org/10.1007/s10822-013-9692-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-013-9692-0