Abstract
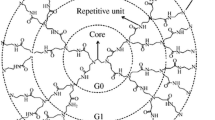
Poly(amidoamine) (PAMAM) dendrimers have been extensively studied as delivery vectors in biomedical applications. A limited number of molecular dynamics (MD) simulation studies have investigated the effect of surface chemistry on therapeutic molecules loading, with the aim of providing insights for biocompatibility improvement and increase in drug loading capacity of PAMAM dendrimers. In this work, fully atomistic MD simulations were employed to study the association of 5-Fluorouracil (5-FU) with amine (NH2)- and hydroxyl (OH)-terminated PAMAM dendrimers of generations 3 and 4 (G3 and G4). MD results show a 1:12, 1:1, 1:27, and 1:4 stoichiometry, respectively, for G3NH2-FU, G3OH-FU, G4NH2-FU, and G4OH-FU complexes, which is in good agreement with the isothermal titration calorimetry results. The results obtained showed that NH2-terminated dendrimers assume segmented open structures with large cavities and more drug molecules can encapsulate inside the dendritic cavities of amine terminated dendrimers. However, OH-terminated have a densely packed structure and therefore, 5-FU drug molecules are more stable to locate close to the surface of the dendrimers. Intermolecular hydrogen bonding analysis showed that 5-FU drug molecules have more tendency to form hydrogen bonds with terminal monomers of OH-terminated dendrimers, while in NH2-terminated these occur both in the inner region and the surface. Furthermore, MM-PBSA analysis revealed that van der Waals and electrostatic energies are both important to stabilize the complexes. We found that drug molecules are distributed uniformly inside the amine and hydroxyl terminated dendrimers and therefore, both dendrimers are promising candidates as drug delivery systems for 5-FU drug molecules.










Similar content being viewed by others
References
Sawant RR, Torchilin VP (2010) Liposomes as ‘smart’ pharmaceutical nanocarriers. Soft Matter 6(17):4026–4044
Peretz S, Regev O (2012) Carbon nanotubes as nanocarriers in medicine. Curr Opin Colloid Interface Sci 17(6):360–368
Elsabahy M, Wooley KL (2012) Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev 41(7):2545–2561
Kesharwani P, Jain K, Jain NK (2014) Dendrimer as nanocarrier for drug delivery. Prog Polym Sci 39(2):268–307
Tomalia DA (2005) Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog Polym Sci 30(3):294–324
Jain V, Bharatam PV (2014) Pharmacoinformatic approaches to understand complexation of dendrimeric nanoparticles with drugs. Nanoscale 6(5):2476–2501
Cakara D, Kleimann J, Borkovec M (2003) Microscopic protonation equilibria of poly (amidoamine) dendrimers from macroscopic titrations. Macromolecules 36(11):4201–4207
Winnicka K, Bielawski K, Rusak M, Bielawska A (2009) The effect of generation 2 and 3 poly (amidoamine) dendrimers on viability of human breast cancer cells. J Health Sci 55(2):169–177
Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T (2003) In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 24(7):1121–1131
Hong S, Leroueil PR, Janus EK, Peters JL, Kober M-M, Islam MT, Orr BG, Baker JR Jr, Banaszak Holl MM (2006) Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjug Chem 17(3):728–734
Leroueil PR, Berry SA, Duthie K, Han G, Rotello VM, McNerny DQ, Baker JR, Orr BG, Banaszak Holl MM (2008) Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Lett 8(2):420–424
Jain K, Kesharwani P, Gupta U, Jain N (2010) Dendrimer toxicity: let’s meet the challenge. Int J Pharma 394(1):122–142
Svenson S (2009) Dendrimers as versatile platform in drug delivery applications. Eur J Pharm Biopharm 71(3):445–462
Cheng Y, Xu Z, Ma M, Xu T (2008) Dendrimers as drug carriers: applications in different routes of drug administration. J Pharm Sci 97(1):123–143
Duncan R, Izzo L (2005) Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev 57(15):2215–2237
Paleos CM, Tsiourvas D, Sideratou Z (2007) Molecular engineering of dendritic polymers and their application as drug and gene delivery systems. Mol Pharm 4(2):169–188
Bielski ER, Zhong Q, Brown M, da Rocha SR (2015) Effect of the conjugation density of triphenylphosphonium cation on the mitochondrial targeting of poly (amidoamine) dendrimers. Mol Pharm 12(8):3043–3053
Zhong Q, Bielski ER, Rodrigues LS, Brown MR, Reineke JJ, da Rocha SR (2016) Conjugation to poly (amidoamine) dendrimers and pulmonary delivery reduce cardiac accumulation and enhance antitumor activity of doxorubicin in lung metastasis. Mol Pharm 13(7):2363–2375
Singh P, Gupta U, Asthana A, Jain NK (2008) Folate and folate—PEG—PAMAM Dendrimers: synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing mice. Bioconjug Chem 19(11):2239–2252
Patri AK, Kukowska-Latallo JF, Baker JR (2005) Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev 57(15):2203–2214
Tomalia DA, Reyna L, Svenson S (2007) Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Portland Press Limited, London
Han M, Chen P, Yang X (2005) Molecular dynamics simulation of PAMAM dendrimer in aqueous solution. Polymer 46(10):3481–3488
Jain V, Maingi V, Maiti PK, Bharatam PV (2013) Molecular dynamics simulations of PPI dendrimer–drug complexes. Soft Matter 9(28):6482–6496
Vergara-Jaque A, Comer J, Monsalve L, González-Nilo FD, Sandoval C (2013) Computationally efficient methodology for atomic-level characterization of dendrimer–drug complexes: a comparison of amine-and acetyl-terminated PAMAM. J Phys Chem B 117(22):6801–6813
Maingi V, Kumar MVS, Maiti PK (2012) PAMAM dendrimer–drug interactions: effect of pH on the binding and release pattern. J Phys Chem B 116(14):4370–4376
Shi X, Lee I, Chen X, Shen M, Xiao S, Zhu M, Baker JR, Wang SH (2010) Influence of dendrimer surface charge on the bioactivity of 2-methoxyestradiol complexed with dendrimers. Soft Matter 6(11):2539–2545
Topp A, Bauer BJ, Tomalia DA, Amis EJ (1999) Effect of solvent quality on the molecular dimensions of PAMAM dendrimers. Macromolecules 32(21):7232–7237
Avila-Salas FN, Sandoval C, Caballero J, Guiñez-Molinos S, Santos LS, Cachau RE, González-Nilo FD (2012) Study of interaction energies between the PAMAM dendrimer and nonsteroidal anti-inflammatory drug using a distributed computational strategy and experimental analysis by ESI-MS/MS. J Phys Chem B 116(7):2031–2039
Tanis I, Karatasos K (2009) Association of a weakly acidic anti-inflammatory drug (ibuprofen) with a poly (amidoamine) dendrimer as studied by molecular dynamics simulations. J Phys Chem B 113(31):10984–10993
Posocco P, Ferrone M, Fermeglia M, Pricl S (2007) Binding at the core. Computational study of structural and ligand binding properties of naphthyridine-based dendrimers. Macromolecules 40(6):2257–2266
Ivanov AA, Jacobson KA (2008) Molecular modeling of a PAMAM-CGS21680 dendrimer bound to an A 2A adenosine receptor homodimer. Bioorg Med Chem Lett 18(15):4312–4315
Evangelista-Lara A, Guadarrama P (2005) Theoretical evaluation of the nanocarrier properties of two families of functionalized dendrimers. Int J Quantum Chem 103(4):460–470
Soto-Castro D, Evangelista-Lara A, Guadarrama P (2006) Theoretical design of dendrimeric fractal patterns for the encapsulation of a family of drugs: salicylanilides. Tetrahedron 62(51):12116–12125
Badalkhani-Khamseh F, Bahrami A, Ebrahim-Habibi A, Hadipour NL (2017) Complexation of nicotinic acid with first generation poly (amidoamine) dendrimers: a microscopic view from density functional theory. Chem Phys Lett 684:103–112
Caballero J, Poblete H, Navarro C, Alzate-Morales JH (2013) Association of nicotinic acid with a poly (amidoamine) dendrimer studied by molecular dynamics simulations. J Mol Graph Model 39:71–78
Yang L, da Rocha SR (2014) PEGylated, NH2-terminated PAMAM dendrimers: a microscopic view from atomistic computer simulations. Mol Pharm 11(5):1459–1470
Schramm OG, López-Cortés X, Santos LS, Laurie VF, Nilo FDG, Krolik M, Fischer R, Di Fiore S (2014) pH-dependent nano-capturing of tartaric acid using dendrimers. Soft Matter 10(4):600–608
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1:19–25
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38
Dennington R, Keith T, Millam J (2009) GaussView, version 5. Semichem Inc, Shawnee
Niu Y, Sun L, Crooks RM (2003) Determination of the intrinsic proton binding constants for poly (amidoamine) dendrimers via potentiometric pH titration. Macromolecules 36(15):5725–5731
Diallo MS, Christie S, Swaminathan P, Balogh L, Shi X, Um W, Papelis C, Goddard WA, Johnson JH (2004) Dendritic chelating agents. 1. Cu (II) binding to ethylene diamine core poly (amidoamine) dendrimers in aqueous solutions. Langmuir 20(7):2640–2651
Pande S, Crooks RM (2011) Analysis of poly (amidoamine) dendrimer structure by UV–Vis spectroscopy. Langmuir 27(15):9609–9613
Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C (2006) Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65(3):712–725
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S (1993) General atomic and molecular electronic structure system. J Comput Chem 14(11):1347–1363
Vanquelef E, Simon S, Marquant G, Garcia E, Klimerak G, Delepine JC, Cieplak P, Dupradeau F-Y (2011) RED Server: a web service for deriving RESP and ESP charges and building force field libraries for new molecules and molecular fragments. Nucl Acids Res 39(suppl_2):W511–W517
Buczkowski A, Waliszewski D, Urbaniak P, Palecz B (2016) Study of the interactions of PAMAM G3-NH 2 and G3-OH dendrimers with 5-fluorouracil in aqueous solutions. Int J Pharm 505(1):1–13
Buczkowski A, Urbaniak P, Palecz B (2012) Thermochemical and spectroscopic studies on the supramolecular complex of PAMAM-NH 2 G4 dendrimer and 5-fluorouracil in aqueous solution. Int J Pharm 428(1):178–182
Buczkowski A, Urbaniak P, Piekarski H, Palecz B (2017) Spectroscopic and calorimetric studies on the interaction between PAMAM G4-OH and 5-fluorouracil in aqueous solutions. Spectrochim Acta Part A 171:401–405
Kumari R, Kumar R, Consortium OSDD, Lynn A (2014) g_mmpbsa A GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inform Model 54(7):1951–1962
Jones CF, Campbell RA, Franks Z, Gibson CC, Thiagarajan G, Vieira-de-Abreu A, Sukavaneshvar S, Mohammad SF, Li DY, Ghandehari H (2012) Cationic PAMAM dendrimers disrupt key platelet functions. Mol Pharm 9(6):1599–1611
Albertazzi L, Gherardini L, Brondi M, Sulis Sato S, Bifone A, Pizzorusso T, Ratto GM, Bardi G (2012) In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol Pharm 10(1):249–260
Liu Y, Bryantsev VS, Diallo MS, Goddard Iii WA (2009) PAMAM dendrimers undergo pH responsive conformational changes without swelling. J Am Chem Soc 131(8):2798–2799
Porcar L, Liu Y, Verduzco R, Hong K, Butler PD, Magid LJ, Smith GS, Chen W-R (2008) Structural investigation of PAMAM dendrimers in aqueous solutions using small-angle neutron scattering: effect of generation. J Phys Chem B 112(47):14772–14778
Prosa TJ, Bauer BJ, Amis EJ, Tomalia DA, Scherrenberg R (1997) A SAXS study of the internal structure of dendritic polymer systems. J Polym Sci Part B 35(17):2913–2924
Rathgeber S, Monkenbusch M, Kreitschmann M, Urban V, Brulet A (2002) Dynamics of star-burst dendrimers in solution in relation to their structural properties. J Chem Phys 117(8):4047–4062
Prosa TJ, Bauer BJ, Amis EJ (2001) From stars to spheres: a SAXS analysis of dilute dendrimer solutions. Macromolecules 34(14):4897–4906
Lee H, Larson RG (2011) Effects of PEGylation on the size and internal structure of dendrimers: self-penetration of long PEG chains into the dendrimer core. Macromolecules 44(7):2291–2298
Lee H, Larson RG (2006) Molecular dynamics simulations of PAMAM dendrimer-induced pore formation in DPPC bilayers with a coarse-grained model. J Phys Chem B 110(37):18204–18211
Maingi V, Jain V, Bharatam PV, Maiti PK (2012) Dendrimer building toolkit: model building and characterization of various dendrimer architectures. J Comput Chem 33(25):1997–2011
Singh BN (2005) A quantitative approach to probe the dependence and correlation of food-effect with aqueous solubility, dose/solubility ratio, and partition coefficient (Log P) for orally active drugs administered as immediate-release formulations. Drug Develop Res 65(2):55–75
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Badalkhani-Khamseh, F., Ebrahim-Habibi, A. & Hadipour, N.L. Atomistic computer simulations on multi-loaded PAMAM dendrimers: a comparison of amine- and hydroxyl-terminated dendrimers. J Comput Aided Mol Des 31, 1097–1111 (2017). https://doi.org/10.1007/s10822-017-0091-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-017-0091-9