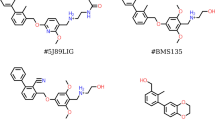
Abstract
The programmed cell death protein 1 (PD-1) and its ligand, PD-L1, constitute an important co-inhibitory immune checkpoint leading to downregulation of immune system. Tumor cells developed a strategy to trigger PD-1/PD-L1 pathway reducing the T cell anticancer activity. Anti-PD-L1 small drugs, generally with improved pharmacokinetic and technological profiles than monoclonal antibodies, became an attractive research topic. Nevertheless, still few works have been published on the chemical features of possible binding sites. In this work, we applied a novel computational protocol based on the combination of the ab initio Fragment Molecular Orbital (FMO) method and a newly developed GRID-DRY approach in order to characterize the PD-L1 binding sites, starting from PD-1/PD-L1 and PD-L1/BMS-ligands (Bristol–Mayers Squibb ligands) complexes. The FMO method allows the calculation of the pair-residues as well as the ligand–residues interactions with ab initio accuracy, whereas the GRID-DRY approach is an effective tool to investigate hydrophobic interactions, not easily detectable by ab initio methods. The present GRID-DRY protocol is able to determine the energy contributions of each ligand atoms to each hydrophobic interaction, both qualitatively and quantitatively. We were also able to identify the three specific hot regions involved in PD-1/PD-L1 protein–protein interaction and in PD-L1/BMS-ligand interactions, in agreement with preceding theoretical/experimental results, and to suggest a specific pharmacophore for PD-L1 inhibitors.











Similar content being viewed by others
Abbreviations
- BMS:
-
Bristol–Mayers Squibb
- DRY:
-
Hydrophobic probe
- Ees :
-
Electrostatic energy
- Eex :
-
Exchange repulsion energy
- Ect :
-
Charge transfer energy
- Edisp :
-
Dispersion energy
- Esolv :
-
Solvation energy
- EEL:
-
Electrostatic energy
- EHB:
-
Hydrogen-bonding energy
- ELJ:
-
Lennard–Jones energy
- ES:
-
Entropic energy
- FMO:
-
Fragment molecular orbitals
- G:
-
Region on PD-L1 surface delimited by Asp26, Asp122, Tyr123, Lys124 and Arg125
- HE:
-
Hydrophobic interaction energy
- HOP:
-
Hybrid orbital projection
- HTRF:
-
Homogenous time resolved fluorescence
- MAO:
-
Mechanism of action
- MIFs:
-
Molecular interaction fields
- N1:
-
Neutral flat N–H probe
- O:
-
Carbonyl oxygen probe
- OH2:
-
Water molecule probe
- PCM:
-
Polarizable continuum model
- PD-1:
-
Programmed cell death protein 1
- PDB:
-
Protein data bank
- PD-L1:
-
Programmed cell death protein 1 ligand
- PIE:
-
Pair interaction energy
- PIEDA:
-
Pair interaction energy decomposition analysis
- PPI:
-
Protein–protein interaction
- P1:
-
Region on PD-L1 surface composed of Tyr56, Glu58, Arg113, Met115 and Tyr123
- P2:
-
Region on PD-L1 surface composed of Met115, Ala121 and Ty123
- QSAR:
-
Quantitative structure−activity relationship
- R2 :
-
Squared Pearson’s correlation coefficient
- RI-MP2:
-
Second-order Møller–Plesset perturbation theory (MP2) gradient with resolution of the identity (RI) approximation
- 3D:
-
Three-dimensional
- SAR:
-
Structure–activity relationship
References
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
Mahoney KM, Freeman GJ, McDermott DF (2015) The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma, PD-1/PD-L1 inhibitors. Clin Ther 37(4):764–782
Blank C, Gajewski TF, Mackensen A (2005) Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 54:307–314
Wilkinson E (2015) Nivolumab success in untreated metastatic melanoma. Lancet Oncol 16:e9
Bagcchi S (2014) Pembrolizumab for treatment of refractory melanoma. Lancet Oncol 15:e419
Lipson EJ, Forde PM, Hammers H, Emens LA, Taube JM, Topolian SL (2015) Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol 42:587–600
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99:12293–12297
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–567
Brahmer JR, Tykodi SS, Chow LQM, Hwu W, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot CH, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465
Philips GK, Atkins M (2015) Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol 27:39–46
Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD (2015) Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26:2375–2391
Chen T, Li Q, Liu Z, Chen Y, Feng F, Sun H (2019) Peptide based and small synthetic molecule inhibitors on PD/PD-L1 pathway: a new choice for immunotherapy? Eur J Med Chem 161:378–398
Zhan M, Hu X, Liu X, Ruan B, Xu J, Liao C (2016) From monoclonal antibodies to small molecules: the development of inhibitors targeting the PD-1/PD-L1 pathway. Drug Discovery Today 21(6):1027–1036
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355
Chowdhury PS, Chamoto K, Honjo T (2018) Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med 283:110–120
Chupak LS, Zheng X (2015) Compounds useful as immunomodulators. WO2015034820A1
Chupak LS, Ding M, Martin SW, Zheng X, Hewawasam P, Connolly TP, Xu N, Yeung K-S, Zhu J, Langley DR, Tenney DJ, Scola PM, Mingo PA (2015) Compounds useful as immunomodulators. WO2015160641
Abdel-Magid AF (2015) Inhibitors of the PD-1/PD-L1 pathway can mobilize the immune system: an innovative potential therapy for cancer and chronic infections. ACS Med Chem Lett 6:489–490
Zak KM, Grudnik P, Guzik K, Zieba BJ, Musielak B, Dömling A, Dubin G, Holak TA (2016) Structural basis for small molecule targeting of the programmed death ligand 1 (PD-L1). Oncotarget 7:30323–30335
Guzik K, Zak KM, Grudnik P, Magiera K, Musielak B, Törner R, Skalniak L, Dömling A, Dubin G, Holak TA (2017) Small-molecule inhibitors of the programmed cell death-1/programmed death-ligand 1 (PD-1/PD-L1) interaction via transiently induced protein states and dimerization of PD-L1. J Med Chem 60(77):5857–5867
Skalniak L, Zak KM, Guzik K, Magiera K, Musielak B, Pachota M, Szelazek B, Kocik J, Grudnik P, Tomala M, Krzanik S, Pyrc K, Dömling A, Dubin G, Holak T (2017) A small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget 8:72167–72181
Sasikumar PGN, Ramachandra M, Naremaddepalli SSS (2015) 1,2,4-Oxadiazole derivatives as immunomodulators. US20150073024
Sasikumar PGN, Ramachandra M, Vadlamani SK, Vemula KR, Satyam LK, Subbarao K, Shrimali RK, Kandepu S (2013) Immunosuppression modulating compounds. EP2585099A2
Sasikumar PGN, Ramachandra M, Naremaddepalli SSS (2013) Peptidomimetic compounds as immunomodulators. WO2013132317A8
Shrimali KR, Subbarao K (2012) Therapeutic compounds for immunomodulation. WO2012168944A1
Miller MM, Mapelli C, Allen MP, Bowsher MS, Boy KM, Gillis EP, Langley DR, Mull E, Poirier MA, Sanghvi N (2014) Macrocyclic inhibitors of the pd-1/pd-l1 and cd80(b7-1)/pd-l1 protein/protein interactions. WO2014151634A1
Sasikumar PGN, Ramachandra M, Naremaddepalli SSS (2015) Cyclic peptidomimetic compounds as immunomodulators. WO2015033303A1
Magiera-Mularz K, Skalniak L, Zak KM, Musielak B, Rudzinska-Szostak E, Berlicki Ł, Kocik J, Grudnik P, Sala D, Zarganes-Tzitzikas T, Shaabani S, Dömling A, Dubin G, Holak TA (2017) Bioactive macrocyclic inhibitors of the PD-1/PD-L1 immune checkpoint. Angew Chemie Int Ed 56:13732–13735
Weinmann H (2016) Cancer immunotherapy: selected targets and small-molecule modulators. ChemMedChem 11:450–466
Guzik K, Tomala M, Muszak D, Konieczny M, Hec A, Błaszkiewicz U, Pustuła M, Butera R, Dömling A, Holak TA (2019) Development of the inhibitors that target the PD-1/PD-L1 interaction—a brief look at progress on small molecules, peptides and macrocycles. Molecules 24:2071
Wells JA, McClendon CL (2007) Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature 450:1001–1009
Arkin RM, Wells JA (2004) Small-molecule inhibitors of protein–protein interactions: progressing towards the dream. Nat Rev Drug Discovery 3:301
Fry DC (2006) Protein–protein interactions as targets for small molecule drug discovery. Biopolymers 84:535–552
Bogan AA, Thorn KS (1998) Anatomy of hot spots in protein interfaces. J Mol Biol 280:1–9
Li J, Liu Q (2009) ‘Double water exclusion’: a hypothesis refining the O-ring theory for the hot spots at protein interfaces. Bioinformatics 25(6):743–750
Moreira IS, Fernandes PA, Ramos MJ (2007) Hot spots—a review of the protein–protein interface determinant amino-acid residues. Proteins 68:803–812
Kortemme T, Baker D (2002) A simple physical model for binding energy hot spots in protein–protein complexes. PNAS 99(22):14116–14121
Morrow JK, Zhang S (2012) Computational prediction of hot spot residues. Curr Pharm Des 18(9):1255–1265
Koes D, Khoury K, Huang Y, Wang W, Bista M, Popowicz GM, Wolf S, Holak TA, Dömling A, Camacho CJ (2012) Enabling large-scale design, synthesis and validation of small molecule protein-protein antagonists. PlosOne 7(3):e32839
Mora JS, Assi SA, Fernandez-Fuentes N (2010) Presaging critical residues in protein interfaces-web server (PCRPi-W): a web server to chart hot spots in protein interfaces. PlosOne 5(8):e12352
Fedorov DG, Nagata T, Kitaura K (2012) Exploring chemistry with the fragment molecular orbital method. Phys Chem Chem Phys 14:7562–7577
Kitaura K, Ikeo E, Asada T, Nakano T, Uebayasi M (1999) Fragment molecular orbital method: an approximate computational method for large molecules. Chem Phys Lett 313:701–706
Nakano T, Kaminuma T, Sato T, Akiyama Y, Uebayasi M, Kitaur K (2000) Fragment molecular orbital method: application to polypeptides. Chem Phys Lett 318:614–618
Nagase K, Kobayashi H, Yoshikawa E, Kurita N (2009) Ab initio molecular orbital calculations on specific interactions between urokinase-type plasminogen activator and its receptor. J Mol Graphics Modell 28:46–53
Paciotti R, Storchi L, Marrone A (2019) An insight of early PrP-E200K aggregation by combined molecular dynamics/fragment molecular orbital approaches. Proteins 87:51–61
Storchi L, Paciotti R, Re N, Marrone A (2015) Investigation of the molecular similarity in closely related protein systems: the PrP case study. Proteins 83:1751–1765
Fukuzawa K, Komeiji Y, Mochizuki Y, Kato A, Nakano T, Tanaka S (2006) Intra- and intermolecular interactions between cyclic-AMP receptor protein and DNA: ab initio fragment molecular orbital study. J Comput Chem 27:948–960
Nemoto T, Fedorov DG, Uebayasi M, Kanazawa K, Kitaura K, Komeiji Y (2005) Ab initio fragment molecular orbital (FMO) method applied to analysis of the ligand–protein interaction in a pheromone-binding protein. Comput Biol Chem 29:434–439
Lim HC, Chun JH, Hwang SB, Kim JW, No KT (2018) Specific interactions of protein-protein interaction between human programmed death 1 (PD-1) and its ligand 1 (PD-L1) with ab initio fragment molecular orbital method. Biophys J 114(3):423A
Lim H, Chun J, Jin X, Kim J, Yoon JH, No KT (2019) Investigation of protein-protein interactions and hot spot region between PD-1 and PD-L1 by fragment molecular orbital method. Sci Rep 9:16727
Goodford PJ (1985) A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J Med Chem 28:849–857
Zak KM, Kitel R, Przetocka S, Golik P, Guzik K, Musielak B, Dömling A, Dubin G, Holak TA (2015) Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure 23:2341–2348
Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W (2013) Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J Comput Aid Mol Des 27(3):221–234
Schrödinger Release 2018–3: Schrödinger Suite 2018–3 Protein Preparation Wizard; Epik, Schrödinger, LLC, New York, NY, 2016 Impact, Schrödinger, LLC, New York, NY, 2016; Prime, Schrödinger, LLC, New York, NY, 2018; Schrödinger Release 2018–3: Prime, Schrödinger, LLC, New York, NY, 2018; Schrödinger Release 2018–3: MacroModel, Schrödinger, LLC, New York, NY, 2018; Schrödinger Release 2018–3: LigPrep Schrödinger LLC, New York, NY (2018)
Jacobson MP, Pincus DL, Rapp CS, Day TJF, Honig B, Shaw DE, Friesner RA (2004) A hierarchical approach to all-atom protein loop prediction. Proteins 55:351–367
Jacobson MP, Friesner RA, Xiang Z, Honig B (2002) On the role of crystal packing forces in determining protein side chain conformations. J Mol Biol 320:597–608
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Gordon MS, Schmidt MW (2005) In: Dykstra CE, Frenking G, Kim KS, Scuseria GE (eds) Theory and applications of computational chemistry: the first forty years. Elsevier, Amsterdam, pp 1167–1189
Ishikawa T, Kuwata K, Ishikawa T, Kuwata K (2009) Fragment molecular orbital calculation using the RI-MP2 method. Chem Phys Lett 474:195–198
Ishikawa T, Kuwata K (2012) RI-MP2 gradient calculation of large molecules using the fragment molecular orbital method. J Phys Chem Lett 3:375–379
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093
Fedorov DG, Kitaura K, Li H, Jensen JH, Gordon MS (2006) The polarizable continuum model (PCM) interfaced with the fragment molecular orbital method (FMO). J Comput Chem 27:976–985
Li H, Fedorov DG, Nagata T, Kitaura K, Jensen JH, Gordon MS (2010) Energy gradients in combined fragment molecular orbital and polarizable continuum model (FMO/PCM) calculation. J Comput Chem 31:778–790
Fedorov DG, Kitaura K (2007) Pair interaction energy decomposition analysis. J Comput Chem 28:222–237
Tanaka S, Mochizuki Y, Komeiji Y, Okiyama Y, Fukuzawa K (2014) Electron-correlated fragment-molecular-orbital calculations for biomolecular and nano systems. Phys Chem Chem Phys 16:10310–10344
Ozawa M, Ozawa T, Ueda K (2017) Application of the fragment molecular orbital method analysis to fragment-based drug discovery of BET (bromodomain and extra-terminal proteins) inhibitors. J Mol Graphics Modell 74:73–82
https://www.moldiscovery.com/soft_grid.php. Accessed February 2019
Von Itzstein M, Wu W, Kok GB, Pegg MS, Dyason JC, Jin B, Phan TV, Smythe ML, White HF, Oliver SW, Colman PM, Varghese JN, Ryan DM, Woods JM, Bethell RC, Hotham VJ, Cameron JM, Penn CR (1993) Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418–423
Milletti F, Storchi L, Sforna G, Cruciani G (2007) New and original pKa prediction method using grid molecular interaction fields. J Chem Inf Model 47:2172–2181
Milletti F, Storchi L, Sforna G, Cross S, Cruciani G (2009) Tautomer enumeration and stability prediction for virtual screening on large chemical databases. J Chem Inf Model 49:68–75
Ahlstrom MM, Ridderströ M, Luthman K, Zamora I (2005) Virtual screening and scaffold hopping based on GRID molecular interaction fields. J Chem Inf Model 45:1313–1323
Bergmann R, Linusson A, Zamora I (2007) SHOP: scaffold hopping by GRID-based similarity searches. J Med Chem 50:2708–2717
Pastor M, Cruciani G, McLay I, Pickett S, Clementi S (2000) Grid-independent descriptors (GRIND): a novel class of alignment-independent three-dimensional molecular descriptors. J Med Chem 43:3233–3243
Cruciani G, Carosati E, De Boeck B, Ethirajulu K, Mackie C, Howe T, Vianello R (2005) MetaSite: understanding metabolism in human cytochromes from the perspective of the chemist. J Med Chem 48:6970–6979
G. Cruciani (ed) Molecular Interaction Fields: Applications in drug discovery and ADME prediction, vol 27, Chapter 1. First published: 26 October 2005 Copyright © 2006 Wiley-VCH Verlag GmbH & Co. KGaA
G. van Rossum (1995) Python tutorial. Technical report CS-R9526, Centrum voor Wiskunde en Informatica (CWI), Amsterdam.
Heifetz A, Chudyk EI, Gleave L, Aldeghi M, Cherezov V, Fedorov DG, Biggin PC, Bodkin MJ (2016) The fragment molecular orbital method reveals new insight into the chemical nature of GPCR−ligand interactions. J Chem Inf Model 56:159–172
Fedorov DG, Kitaura K (2016) Subsystem analysis for the fragment molecular orbital method and its application to protein−ligand binding in solution. J Phys Chem A 120:2218–2231
Sunshine J, Taube JM (2015) PD-1/PD-L1 inhibitors. Curr Opin Pharmacol 23:32–38
Sun X, Liang L, Gu J, Zhuo W, Yan X, Xie T, Wu Z, Liu X, Gou X, Liu W, He G, Gan Y, Chang S, Shi H, Hu J (2019) Inhibition of programmed cell death protein ligand-1 (PD-L1) by benzyl ether derivatives: analyses of conformational change, molecular recognition and binding free energy. J Biomol Struct Dyn 37(18):4801–4812
Pascolutti R, Sun X, Kao J, Maute RL, Ring AM, Bowman GR, Kruse AC (2016) Structure and dynamics of PD-L1 and an ultra-high-affinity PD-1 receptor mutant. Structure 24:1719–1728
Shi D, Zhou S, Liu X, Zhao C, Liu H, Yao X (2018) Understanding the structural and energetic basis of PD-1 and monoclonal antibodies bound to PD-L1: a molecular modeling perspective. BBA General Subjects 1862:576–588
Ding H, Liu H (2019) Mapping the binding hot spots on human programmed cell death 1 and its ligand with free-energy simulations. J Chem Inf Model. https://doi.org/10.1021/acs.jcim.9b00337
Perry E, Mills JJ, Zhao B, Wang F, Sun Q, Christov PP, Tarr JC, Rietz TA, Olejniczak ET, Lee T, Fesik S (2019) Fragment-based screening of programmed death ligand 1 (PD-L1). Bioorg Med Chem Lett 29(6):786–790
Mejías C, Guirola O (2019) Pharmacophore model of immunocheckpoint protein PD-L1 by cosolvent molecular dynamics simulations. J Mol Graphics Modell 91:105–111
Eyrisch S, Helms V (2009) What induces pocket openings on protein surface patches involved in protein–protein interactions? J Comput Aid Mol Des 23:73–86
Guo W, Wisniewski JA, Ji H (2014) Hot spot-based design of small-molecule inhibitors for protein–protein interactions. Bioorg Med Chem Lett 24:2546–2554
Metz A, Pfleger C, Kopitz H, Pfeiffer-Marek S, Baringhaus K, Gohlke H (2011) Hot spots and transient pockets: predicting the determinants of small-molecule binding to a protein-protein interface. J Chem Inf Model 52:120–133
Stank A, Kokh DB, Fuller JC, Wade RC (2016) Protein binding pocket dynamics. Acc Chem Res 49:809–815
Acknowledgement
We thank the Ministry of Education, University and Research (MIUR) for financial support. Moreover, we are thankful to Prof. Alessandro Marrone for his suggestions and useful discussions.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paciotti, R., Agamennone, M., Coletti, C. et al. Characterization of PD-L1 binding sites by a combined FMO/GRID-DRY approach. J Comput Aided Mol Des 34, 897–914 (2020). https://doi.org/10.1007/s10822-020-00306-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-020-00306-0