Abstract
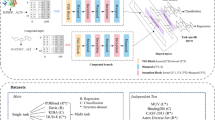
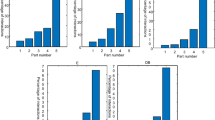
In the field of drug–target interactions prediction, the majority of approaches formulated the problem as a simple binary classification task. These methods used binary drug–target interaction datasets to train their models. The prediction of drug–target interactions is inherently a regression problem and these interactions would be identified according to the binding affinity between drugs and targets. This paper deals the binary drug–target interactions and tries to identify the binary interactions based on the binding strength of a drug and its target. To this end, we propose a semi-supervised transfer learning approach to predict the binding affinity in a continuous spectrum for binary interactions. Due to the lack of training data with continuous binding affinity in the target domain, the proposed method makes use of the information available in other domains (i.e. source domain), via the transfer learning approach. The general framework of our algorithm is based on an objective function, which considers the performance in both source and target domains as well as the unlabeled data in the target domain via a regularization term. To optimize this objective function, we make use of a gradient boosting machine which constructs the final model. To assess the performance of the proposed method, we have used some benchmark datasets with binary interactions for four classes of human proteins. Our algorithm identifies interactions in a more realistic situation. According to the experimental results, our regression model performs better than the state-of-the-art methods in some procedures.






Similar content being viewed by others
Data availability
The target datasets used during the current study are available in the: http://web.kuicr.kyoto-u.ac.jp/supp/yoshi/drugtarget/. The source dataset used in this study is available in: https://www.bindingdb.org/bind/index.jsp.
References
Macalino SJY, Gosu V, Hong S, Choi S (2015) Role of computer-aided drug design in modern drug discovery. Arch Pharmacol Res 38(9):1686–1701. https://doi.org/10.1007/s12272-015-0640-5
Ezzat A, Wu M, Li X-L, Kwoh C-K (2018) Computational prediction of drug–target interactions using chemogenomic approaches: an empirical survey. Brief Bioinform. https://doi.org/10.1093/bib/bby002
Thafar M, Raies AB, Albaradei S, Essack M, Bajic VB (2019) Comparison study of computational prediction tools for drug-target binding affinities. Front Chem 7:782–782. https://doi.org/10.3389/fchem.2019.00782
Bagherian M, Sabeti E, Wang K, Sartor MA, Nikolovska-Coleska Z, Najarian K (2021) Machine learning approaches and databases for prediction of drug–target interaction: a survey paper. Brief Bioinform 22(1):247–269. https://doi.org/10.1093/bib/bbz157
Chen R, Liu X, Jin S, Lin J, Liu J (2018) Machine learning for drug–target interaction prediction. Molecules. https://doi.org/10.3390/molecules23092208
Sachdev K, Gupta MK (2019) A comprehensive review of feature based methods for drug target interaction prediction. J Biomed Inform 93:103159. https://doi.org/10.1016/j.jbi.2019.103159
Öztürk H, Özgür A, Ozkirimli E (2018) DeepDTA: deep drug–target binding affinity prediction. Bioinformatics 34(17):i821–i829. https://doi.org/10.1093/bioinformatics/bty593
Pahikkala T et al (2015) Toward more realistic drug–target interaction predictions. Brief Bioinform 16(2):325–337. https://doi.org/10.1093/bib/bbu010
Gilson MK, Liu T, Baitaluk M, Nicola G, Hwang L, Chong J (2016) BindingDB in 2015: a public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucl Acids Res 44(D1):D1045–D1053. https://doi.org/10.1093/nar/gkv1072
Dahl G, Akerud T (2013) Pharmacokinetics and the drug–target residence time concept. Drug Discov Today 18(15–16):697–707. https://doi.org/10.1016/j.drudis.2013.02.010
He T, Heidemeyer M, Ban F, Cherkasov A, Ester M (2017) SimBoost: a read-across approach for predicting drug–target binding affinities using gradient boosting machines. J Cheminform 9(1):24–24. https://doi.org/10.1186/s13321-017-0209-z
Abbasi K, Razzaghi P, Poso A, Amanlou M, Ghasemi JB, Masoudi-Nejad A (2020) DeepCDA: deep cross-domain compound–protein affinity prediction through LSTM and convolutional neural networks. Bioinformatics 36(17):4633–4642. https://doi.org/10.1093/bioinformatics/btaa544
Jiang M et al (2020) Drug–target affinity prediction using graph neural network and contact maps. RSC Adv 10(35):20701–20712. https://doi.org/10.1039/D0RA02297G
Bleakley K, Yamanishi Y (2009) Supervised prediction of drug–target interactions using bipartite local models. Bioinformatics 25(18):2397–2403. https://doi.org/10.1093/bioinformatics/btp433
Mei JP, Kwoh CK, Yang P, Li XL, Zheng J (2013) Drug-target interaction prediction by learning from local information and neighbors. Bioinformatics 29(2):238–245. https://doi.org/10.1093/bioinformatics/bts670
Nascimento AC, Prudencio RB, Costa IG (2016) A multiple kernel learning algorithm for drug-target interaction prediction. BMC Bioinform 17:46. https://doi.org/10.1186/s12859-016-0890-3
van Laarhoven T, Nabuurs SB, Marchiori E (2011) Gaussian interaction profile kernels for predicting drug–target interaction. Bioinformatics 27(21):3036–3043. https://doi.org/10.1093/bioinformatics/btr500
Ding Y, Tang J, Guo F (2020) Identification of drug–target interactions via dual laplacian regularized least squares with multiple kernel fusion. Knowl Based Syst 204:106254. https://doi.org/10.1016/j.knosys.2020.106254
Gönen M (2012) Predicting drug–target interactions from chemical and genomic kernels using Bayesian matrix factorization. Bioinformatics 28(8):2304–2310. https://doi.org/10.1093/bioinformatics/bts360
Zheng X, Ding H, Mamitsuka H, Zhu S (2013) Collaborative matrix factorization with similarities for predicting drug-target interactions. In: Presented at the Proceedings of the 19th ACM SIGKDD international conference on Knowledge discovery and data mining, Chicago, IL, 2013. [Online]. Available: https://doi.org/10.1145/2487575.2487670
Liu Y, Wu M, Miao C, Zhao P, Li X-L (2016) Neighborhood regularized logistic matrix factorization for drug–target interaction prediction. PLoS Comput Biol 12(2):e1004760–e1004760. https://doi.org/10.1371/journal.pcbi.1004760
Ezzat A, Zhao P, Wu M, Li XL, Kwoh CK (2017) Drug–target interaction prediction with graph regularized matrix factorization. IEEE/ACM Trans Comput Biol Bioinform 14(3):646–656. https://doi.org/10.1109/tcbb.2016.2530062
Cui Z, Gao Y-L, Liu J-X, Dai L-Y, Yuan S-S (2019) L2,1-GRMF: an improved graph regularized matrix factorization method to predict drug-target interactions. BMC Bioinform 20(8):287. https://doi.org/10.1186/s12859-019-2768-7
Rayhan F, Ahmed S, Farid DM, Dehzangi A, Shatabda S (2019) CFSBoost: cumulative feature subspace boosting for drug–target interaction prediction. J Theor Biol 464:1–8
Rayhan F et al (2017) iDTI-ESBoost: identification of drug target interaction using evolutionary and structural features with boosting. Sci Rep 7(1):17731. https://doi.org/10.1038/s41598-017-18025-2
Rayhan F, Ahmed S, Mousavian Z, Farid DM, Shatabda S (2020) FRnet-DTI: deep convolutional neural network for drug-target interaction prediction. Heliyon 6(3):e03444–e03444. https://doi.org/10.1016/j.heliyon.2020.e03444
Xia Z, Wu LY, Zhou X, Wong ST (2010) Semi-supervised drug-protein interaction prediction from heterogeneous biological spaces. BMC Syst Biol 4(Suppl 2):S6. https://doi.org/10.1186/1752-0509-4-s2-s6
Keum J, Nam H (2017) SELF-BLM: prediction of drug-target interactions via self-training SVM. PLoS ONE 12(2):e0171839. https://doi.org/10.1371/journal.pone.0171839
Davis MI et al (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 29(1):1046–51. https://doi.org/10.1038/nbt.1990
Zhuang F et al (2021) A comprehensive survey on transfer learning. Proc IEEE 109(1):43–76. https://doi.org/10.1109/JPROC.2020.3004555
Pan SJ, Yang Q (2010) A survey on transfer learning. IEEE Trans Knowl Data Eng 22(10):1345–1359. https://doi.org/10.1109/tkde.2009.191
Gao J, Fan W, Jiang J, Han J (2008) Knowledge transfer via multiple model local structure mapping. In: Proceedings of the 14th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Conference Proceedings, pp 283–291
Richardson M, Domingos P (2006) Markov logic networks. Mach Learn 62(1):107–136. https://doi.org/10.1007/s10994-006-5833-1
Mihalkova, L, Huynh T, Mooney RJ (2007) Mapping and revising Markov logic networks for transfer learning. In: Proceedings of the 22nd national conference on Artificial intelligence - Volume 1). Vancouver, BC, AAAI Press, pp 608–614
Zou N, Baydogan M, Zhu Y, Wang W, Zhu J, Li J (2015) A transfer learning approach for predictive modeling of degenerate biological systems. Technometrics 57(3):362–373. https://doi.org/10.1080/00401706.2015.1044117
Mieth B et al (2019) Sing transfer learning from prior reference knowledge to improve the clustering of single-cell RNA-Seq data. Sci Rep. https://doi.org/10.1038/s41598-019-56911-z
Turki T, Wei Z, Wang JTL (2017) Transfer learning approaches to improve drug sensitivity prediction in multiple myeloma patients. IEEE Access 5:7381–7393
Mourragui S, Loog M, van de Wiel MA, Reinders MJT, Wessels LFA (2019) PRECISE: a domain adaptation approach to transfer predictors of drug response from pre-clinical models to tumors. Bioinformatics 35(14):i510–i519. https://doi.org/10.1093/bioinformatics/btz372
Friedman JH (2001) Greedy function approximation: a gradient boosting machine. J Ann Stat 29:1189–1232. https://doi.org/10.1214/aos/1013203451
Ganjisaffar Y, Caruana R, Lopes CV (2011) Bagging gradient-boosted trees for high precision, low variance ranking models. In: Presented at the Proceedings of the 34th international ACM SIGIR conference on Research and development in Information Retrieval, Beijing, China
Natekin A, Knoll A (2013) Gradient boosting machines, a tutorial. Front Neurorobot 7:21–21. https://doi.org/10.3389/fnbot.2013.00021
Yamanishi Y, Araki M, Gutteridge A, Honda W, Kanehisa M (2008) Prediction of drug–target interaction networks from the integration of chemical and genomic spaces. Bioinformatics 24(13):i232–i240. https://doi.org/10.1093/bioinformatics/btn162
Weiss K, Khoshgoftaar TM, Wang D (2016) A survey of transfer learning. J Big Data 3(1):9. https://doi.org/10.1186/s40537-016-0043-6
Kim S et al (2019) PubChem 2019 update: improved access to chemical data. Nucl Acids Res 47(D1):D1102–D1110. https://doi.org/10.1093/nar/gky1033
Vidal D, Thormann M, Pons M (2005) LINGO, an efficient holographic text based method to calculate biophysical properties and intermolecular similarities. J Chem Inf Model 45(2):386–393. https://doi.org/10.1021/ci0496797
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Rouge (in French), Lausanne
Smith TF, Waterman MS (1981) Identification of common molecular subsequences. J Mol Biol 147(1):195–197. https://doi.org/10.1016/0022-2836(81)90087-5
Wishart DS et al (2018) DrugBank 5.0: a major update to the DrugBank database for 2018. Nucl Acids Res 46(D1):D1074–D1082. https://doi.org/10.1093/nar/gkx1037
Hecker N et al (2012) SuperTarget goes quantitative: update on drug-target interactions. Nucl Acids Res 40(Database issue):1113–1117. https://doi.org/10.1093/nar/gkr912
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K (2016) KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucl Acids Res 45(D1):D353–D361. https://doi.org/10.1093/nar/gkw1092
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have not any financial and personal relationships with other people or organizations that could inappropriately influence this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanoori, B., Zolghadri Jahromi, M. & Mansoori, E.G. Binding affinity prediction for binary drug–target interactions using semi-supervised transfer learning. J Comput Aided Mol Des 35, 883–900 (2021). https://doi.org/10.1007/s10822-021-00404-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-021-00404-7