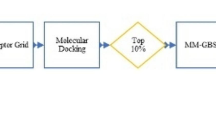
Abstract
In this work, the ab initio fragment molecular orbital (FMO) method was applied to calculate and analyze the binding energy of two biscarbene-Au(I) derivatives, [Au(9-methylcaffein-8-ylidene)2]+ and [Au(1,3-dimethylbenzimidazol-2-ylidene)2]+, to the DNA G-Quadruplex structure. The FMO2 binding energy considers the ligand-receptor complex as well as the isolated forms of energy-minimum state of ligand and receptor, providing a better description of ligand-receptor affinity compared with simple pair interaction energies (PIE). Our results highlight important features of the binding process of biscarbene-Au(I) derivatives to DNA G-Quadruplex, indicating that the total deformation-polarization energy and desolvation penalty of the ligands are the main terms destabilizing the binding. The pair interaction energy decomposition analysis (PIEDA) between ligand and nucleobases suggest that the main interaction terms are electrostatic and charge-transfer energies supporting the hypothesis that Au(I) ion can be involved in π-cation interactions further stabilizing the ligand-receptor complex. Moreover, the presence of polar groups on the carbene ring, as C = O, can improve the charge-transfer interaction with K+ ion. These findings can be employed to design new powerful biscarbene-Au(I) DNA-G quadruplex binders as promising anticancer drugs. The procedure described in this work can be applied to investigate any ligand-receptor system and is particularly useful when the binding process is strongly characterized by polarization, charge-transfer and dispersion interactions, properly evaluated by ab initio methods.







Similar content being viewed by others
Data availability
The 3D coordinates of Gq, Gq-13, Gq-1(I), Gq-1(II), Gq-1(III), Gq-2(I), Gq-1(II), Gq-2(III), ligands 1 and 2 structures reported in this study are also available in the pdb and xyz file formats at the following link https://doi.org/10.5281/zenodo.7102260.
Abbreviations
- CADD:
-
Computer-assisted drug discovery
- Gq:
-
G-quadruplex
- DNA:
-
Deoxyribonucleic acid;
- FF:
-
Force field
- FE:
-
Fragment efficiency
- FMO:
-
Fragment molecular orbital method
- FRET:
-
Fluorescence resonance energy transfer assay
- F2LE:
-
FMO2 ligand efficiency
- L:
-
Ligand
- LR:
-
Ligand-receptor complex
- MD:
-
Molecular dynamics
- MEP:
-
Molecular electrostatic potential
- MM:
-
Molecular mechanics
- MM/GBSA:
-
Molecular mechanics/generalized Born surface area method
- MM/PBSA:
-
Molecular mechanics/ Poisson-Boltzmann surface area method
- PIE:
-
Pair interaction energy
- PIEDA:
-
Pair interaction energy decomposition analysis
- QM:
-
Quantum mechanics
- R:
-
Receptor
References
Leelananda SP, Lindert S (2016) Computational methods in drug discovery. Beilstein J Org Chem 12:2694–2718. https://doi.org/10.3762/bjoc.12.267
Jorgensen WL (2004) The many roles of computation in drug discovery. Science 303:1813–1818. https://doi.org/10.1126/science.1096361
Vanommeslaeghe K, Guvench O, MacKerell DA Jr (2014) Molecular mechanics. Curr Pharm Des 20:3281–3292
Durrant JD, McCammon JA (2011) Molecular dynamics simulations and drug discovery. BMC Biol 9:71. https://doi.org/10.1186/1741-7007-9-71
Abel R, Wang L, Harder ED, Berne BJ, Friesner RA (2017) Advancing drug discovery through enhanced free energy calculations. Acc Chem Res 50:1625–1632. https://doi.org/10.1021/acs.accounts.7b00083
Meng X, Zhang H, Mezei M, Cui M (2011) Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des 7:146–157. https://doi.org/10.2174/157340911795677602
Genheden S, Ryde U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10:449–461. https://doi.org/10.1517/17460441.2015.1032936
Cole DJ, Horton JT, Nelson L, Kurdekar V (2019) The future of force fields in computer-aided drug design. Future Med Chem 11:2359–2363. https://doi.org/10.4155/fmc-2019-0196
Fernádez I, Cossío FP (2014) Applied computational chemistry. Chem Soc Rev 43:4906–4908. https://doi.org/10.1039/C4CS90040E
Krylov A, Windus TL, Barnes T, Marin-Rimoldi E, Nash JA, Pritchard B, Smith DGA, Altarawy D, Saxe P, Clementi C, Crawford TD, Harrison RJ, Jha S, Pande VS, Head-Gordon T (2018) Perspective: computational chemistry software and its advancement as illustrated through three grand challenge cases for molecular science. J Chem Phys 149:180901. https://doi.org/10.1063/1.5052551
Friesner RA (2005) Ab initio quantum chemistry: methodology and applications. PNAS 102:6648–6653. https://doi.org/10.1073/pnas.0408036102
Grimme S, Schreiner PR (2018) Computational chemistry: the fate of current methods and future challenges. Angew Chem Int Ed 57:4170–4176. https://doi.org/10.1002/anie.201709943
Friesner RA (2004) Combined quantum and molecular mechanics (QM/MM). Drug Discov Today Technol 1:253–260. https://doi.org/10.1016/j.ddtec.2004.11.008
Fedorov DG, Kitaura K (2007) Extending the power of quantum chemistry to large systems with the fragment molecular orbital method. J Phys Chem A 111:6904–6914. https://doi.org/10.1021/jp0716740
Steinmann C, Fedorov DG, Jensen JH (2010) Effective fragment molecular orbital method: a merger of the effective fragment potential and fragment molecular orbital methods. J Phys Chem A 114(33):8705–8712. https://doi.org/10.1021/jp101498m
Fedorov DG, Kitaura K (2006) The three-body fragment molecular orbital method for accurate calculations of large systems. Chem Phys Lett 433:182–187. https://doi.org/10.1016/j.cplett.2006.10.052
Nakano T, Mochizuki Y, Yamashita K, Watanabe C, Fukuzawa K, Segawa K, Okiyama Y, Tsukamoto T, Tanaka S (2012) Development of the four-body corrected fragment molecular orbital (FMO4) method. Chem Phys Lett 523:128–133. https://doi.org/10.1016/j.cplett.2011.12.004
Fedorov DG, Kitaura K (2007) Pair interaction energy decomposition analysis. J Comput Chem 28:222–237. https://doi.org/10.1002/jcc.20496
Fedorov DG, Kitaura K (2012) Energy decomposition analysis in solution based on the fragment molecular orbital method. J Phys Chem A 116:704–719. https://doi.org/10.1021/jp209579w
Ma B, Yamaguchi K, Fukuoka M, Kuwata K (2016) Logical design of anti-prion agents using NAGARA. Biochem Biophys Res Commun 469:930–935. https://doi.org/10.1016/j.bbrc.2015.12.106
Paciotti R, Agamennone M, Coletti C, Storchi L (2020) Characterization of PD-L1 binding sites by a combined FMO/GRID-DRY approach. J Comput Aided Mol Des 34:897–914. https://doi.org/10.1007/s10822-020-00306-0
Watanabe C, Watanabe H, Fukuzawa K, Parker LJ, Okiyama Y, Yuki H, Yokoyama S, Nakano H, Tanaka S, Honma T (2017) Theoretical analysis of activity cliffs among benzofuranone-class Pim1 inhibitors using the fragment molecular orbital method with molecular mechanics poisson−Boltzmann surface area (FMO+MMPBSA) approach. J Chem Inf Model 57:2996–3010. https://doi.org/10.1021/acs.jcim.7b00110
Heifetz A, Chudyk EI, Gleave L, Aldeghi M, Cherezov V, Fedorov DG, Biggin PC, Bodkin MJ (2016) The fragment molecular orbital method reveals new insight into the chemical nature of GPCR−ligand interactions. J Chem Inf Model 56:159–172. https://doi.org/10.1021/acs.jcim.5b00644
Fedorov DG, Kitaura K (2016) Subsystem analysis for the fragment molecular orbital method and its application to protein−ligand binding in solution. J Phys Chem A 120:2218–2231. https://doi.org/10.1021/acs.jpca.6b00163
Rhodes D, Lipps HJ (2015) G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res 43(18):8627–8637. https://doi.org/10.1093/nar/gkv862
Lightfoot HL, Hagen T, Tatum NJ, Hall J (2019) The diverse structural landscape of quadruplexes. FEBS Lett 593:2083–2102. https://doi.org/10.1002/1873-3468.13547
de Luzuriaga IO, Lopez X, Gil A (2021) Learning to model G-Quadruplexes: current methods and perspectives. Annu Rev Biophys 50:209–243. https://doi.org/10.1146/annurev-biophys-060320-091827
Terenzi A, Bonsignore R, Spinello A, Gentile C, Martorana A, Ducani C, Högberg B, Almerico AM, Lauria A, Barone G (2014) Selective G-quadruplex stabilizers: Schiff-base metal complexes with anticancer activity. RSC Adv 4:33245–33256. https://doi.org/10.1039/C4RA05355A
Karim NHA, Mendoza O, Shivalingam A, Thompson AJ, Ghosh S, Kuimova MK, Vilar R (2014) Salphen metal complexes as tunable G-quadruplex binders and optical probes. RSC Adv 4:3355–3363. https://doi.org/10.1039/C3RA44793F
Keating LR, Szalai VA (2004) Parallel-stranded guanine quadruplex interactions with a copper cationic porphyrin. Biochemistry 43:15891–15900. https://doi.org/10.1021/bi0483209
Kieltyka R, Englebienne P, Fakhoury J, Autexier C, Moitessier N, Sleiman HF (2008) A platinum supramolecular square as an effective G-Quadruplex binder and telomerase inhibitor. J Am Chem Soc 130:10040–10041. https://doi.org/10.1021/ja8014023
Wu P, Ma DL, Leung CH, Yan SC, Zhu N, Abagyan R, Che C (2009) Stabilization of G-quadruplex DNA with platinum(II) Schiff base complexes: luminescent probe and down-regulation of C-Myc oncogene expression. Chem Eur J 15:13008–13021. https://doi.org/10.1002/chem.200901943
Terenzi A, Lötsch D, van Schoonhoven S, Roller A, Kowol CR, Berger W, Keppler BK, Barone G (2016) Another step toward DNA selective targeting: NiII and CuII complexes of a Schiff base ligand able to bind gene promoter G-quadruplexes. Dalton Trans 45:7758–7767. https://doi.org/10.1039/C6DT00648E
Xia Y, Chen Q, Qin X, Sun D, Zhang J, Liu J (2013) Studies of ruthenium(II)-2,20-bisimidazole complexes on binding to G-quadruplex DNA and inducing apoptosis in HeLa cells. New J Chem 37:3706–3715. https://doi.org/10.1039/C3NJ00542A
Chen X, Wu JH, Lai YW, Zhao R, Chao H, Ji LN (2013) Targeting telomeric G-quadruplexes with the ruthenium(II) complexes [Ru(bpy)2(ptpn)]2+ and [Ru(phen)2(ptpn)]2+. Dalton Trans 42:4386–4397. https://doi.org/10.1039/C3DT32921F
Tuntiwechapikul W, Lee JT, Salazar M (2001) Design and synthesis of the G-quadruplex-specific cleaving reagent perylene-EDTA•iron(II). J Am Chem Soc 123:5606–5607. https://doi.org/10.1021/ja0156439
Tuntiwechapikul W, Salazar M (2001) Cleavage of telomeric G-quadruplex DNA with perylene-EDTA•Fe(II). Biochemistry 40:13652–13658. https://doi.org/10.1021/bi011363u
Prato G, Silvent S, Saka S, Lamberto M, Kosenkov D (2015) Thermodynamics of binding of Di- and tetrasubstituted naphthalene diimide ligands to DNA G-quadruplex. J Phys Chem B 119:3335–3347. https://doi.org/10.1021/jp509637y
Neidle S (2017) Quadruplex nucleic acids as targets for anticancer therapeutics. Nat Rev Chem 1:0041. https://doi.org/10.1038/s41570-017-0041
Tassinari M, Cimino-Reale G, Nadai M, Doria F, Butovskaya E, Recagni M, Freccero M, Zaffaroni N, Richter SN, Folini M (2018) Down-regulation of the androgen receptor by G-quadruplex ligands sensitizes castration-resistant prostate cancer cells to enzalutamide. J Med Chem 61:8625–8638. https://doi.org/10.1021/acs.jmedchem.8b00502
Brooks TA, Kendrick S, Hurley L (2010) Making sense of G-quadruplex and i-motif functions in oncogene promoters. FEBS J 277:3459–3469. https://doi.org/10.1111/j.1742-4658.2010.07759.x
Lago S, Nadai M, Ruggiero E, Tassinari M, Marušič M, Tosoni B, Frasson I, Cernilogar FM, Pirota V, Doria F, Plavec J, Schotta G, Richter SN (2021) The MDM2 inducible promoter folds into four-tetrad antiparallel G-quadruplexes targetable to fight malignant liposarcoma. Nucleic Acids Res 49:847–863. https://doi.org/10.1093/nar/gkaa1273
Bertrand B, Stefan L, Pirrotta M, Monchaud D, Bodio E, Richard P, Le Gendre P, Warmerdam E, de Jager MH, Groothuis GMM, Picquet M, Casini A (2014) Caffeine-based gold(I) N-heterocyclic carbenes as possible anticancer agents: synthesis and biological properties. Inorg Chem 53:2296–2303. https://doi.org/10.1021/ic403011h
Stefan L, Bertrand B, Richard P, Le Gendre P, Denat F, Picquet M, Monchaud D (2012) Assessing the differential affinity of small molecules for noncanonical DNA structures. ChemBioChem 13:1905–1912. https://doi.org/10.1002/cbic.201200396
Bazzicalupi C, Ferraroni M, Papi F, Massai L, Bertrand B, Messori L, Gratteri P, Casini A (2016) Determinants for tight and selective binding of a medicinal dicarbene gold(I) complex to a telomeric DNA G-quadruplex: a joint ESI MS and XRD investigation. Angew Chem 128:4328–4331. https://doi.org/10.1002/ange.201511999
Guarra F, Marzo T, Ferraroni M, Papi F, Bazzicalupi C, Gratteri P, Pescitelli G, Messori L, Biver T, Gabbiani C (2018) Interaction of a gold(I) dicarbene anticancer drug with human telomeric DNA G-quadruplex: solution and computationally aided X-ray diffraction analysis. Dalton Trans 47:16132–16138. https://doi.org/10.1039/C8DT03607A
Nayis A, Liebl K, Frost CV, Zacharias M (2021) Targeting telomeres: molecular dynamics and free energy simulation of gold-carbene binding to DNA. Biophys J 120:101–108. https://doi.org/10.1016/j.bpj.2020.11.2263
Storchi L, Paciotti R, Re N, Marrone A (2015) Investigation of the molecular similarity in closely related protein systems: the PrP case study. Proteins 83:1751–1765. https://doi.org/10.1002/prot.24836
Paciotti R, Storchi L, Marrone A (2019) An insight of early PrP-E200K aggregation by combined molecular dynamics/fragment molecular orbital approaches. Proteins 87:51–61. https://doi.org/10.1002/prot.25621
Nagase K, Kobayashi H, Yoshikawa E, Kurita N (2009) Ab initio molecular orbital calculations on specific interactions between urokinase-type plasminogen activator and its receptor. J Mol Graph Model 28:46–53. https://doi.org/10.1016/j.jmgm.2009.04.001
Kurisaki I, Fukuzawa K, Komeiji Y, Mochizuki Y, Nakano T, Imada J, Chmielewski A, Rothstein SM, Watanabe H, Tanaka S (2007) Visualization analysis of inter-fragment interaction energies of CRP–cAMP–DNA complex based on the fragment molecular orbital method. Biophys Chem 130:1–9. https://doi.org/10.1016/j.bpc.2007.06.011
Yoshino R, Yasuo N, Inaoka DK, HagiwaraY OK, Orita M, Inoue M, Shiba T, Harada S, Honma T, Balogun EO, da Rocha JR, Montanari CA, Kita K, Sekijima M (2015) Pharmacophore modeling for anti-chagas drug design using the fragment molecular orbital method. PLoS ONE 10(5):1–15. https://doi.org/10.1371/journal.pone.0125829
Fedorov DG, Kitaura K, Li H, Jensen JH, Gordon MS (2006) The polarizable continuum model (PCM) interfaced with the fragment molecular orbital method (FMO). J Comput Chem 27:976–985. https://doi.org/10.1002/jcc.20406
Ozawa M, Ozawa T, Ueda K (2017) Application of the fragment molecular orbital method analysis to fragment-based drug discovery of BET (bromodomain and extra-terminal proteins) inhibitors. J Mol Graph Model 74:73–82. https://doi.org/10.1016/j.jmgm.2017.02.013
Alexeev Y, Mazanetz MP, Ichihara O, Fedorov DG (2012) GAMESS as a free quantum-mechanical platform for drug research. Curr Top Med Chem 12:2013–2033. https://doi.org/10.2174/156802612804910269
Hopkins AL, Keserü GM, Leeson PD, Rees DC, Reynolds CH (2014) The role of ligand efficiency metrics in drug discovery. Nat Rev Drug Discovery 13:105–121. https://doi.org/10.1038/nrd4163
Abad-Zapatero C (2007) Ligand efficiency indices for effective drug discovery. Expert Opin Drug Discov 2(4):469–488. https://doi.org/10.1517/17460441.2.4.469
Mazanetz MP, Ichihara O, Law RJ, Whittaker M (2011) Prediction of cyclin-dependent kinase 2 inhibitor potency using the fragment molecular orbital method. J Cheminform 3(2):1–15. https://doi.org/10.1186/1758-2946-3-2
Otsuka T, Okimoto N, Taiji M (2015) Assessment and acceleration of binding energy calculations for protein-ligand complexes by the fragment molecular orbital method. J Comput Chem 36:2209–2218. https://doi.org/10.1002/jcc.24055
Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aid Mol Des 27(3):221–234. https://doi.org/10.1007/s10822-013-9644-8
Schrödinger Release 2018–3: Schrödinger suite 2018–3 protein preparation Wizard; LLC, New York, NY, 2018; Schrödinger Release 2018–3: MacroModel, Schrödinger, LLC, New York, NY, 2018
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA (2009) Gaussian 09. Revision D.01. Gaussian Inc., Wallingford
Bursch M, Neugebauer H, Grimme S (2019) Structure optimisation of large transition-metal complexes with extended tight-binding methods. Angew. Chem. Int. Ed. 58:11078–11087. https://doi.org/10.1002/anie.201904021
Grimme S, Bannwarth C, Shushkov P (2017) A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parameterized for all spd-block elements (Z = 1–86). J Chem Theory Comput 13:1989–2009. https://doi.org/10.1021/acs.jctc.7b00118
Bannwarth C, Ehlert S, Grimme S (2019) GFN2-xTB-An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J Chem Theory Comput 15:1652–1671. https://doi.org/10.1021/acs.jctc.8b01176
Ishikawa T, Kuwata K (2012) RI-MP2 gradient calculation of large molecules using the fragment molecular orbital method. J Phys Chem Lett 3:375–379. https://doi.org/10.1021/jz201697x
Ishikawa T, Kuwata K (2009) Fragment molecular orbital calculation using the RI-MP2 method. Chem Phys Lett 474:195–198. https://doi.org/10.1016/j.cplett.2009.04.045
Rahalkar AP, Katouda M, Gadre SR, Nagase S (2010) Molecular tailoring approach in conjunction with MP2 and RI-MP2 codes: a comparison with fragment molecular orbital method. J Comput Chem 31:2405–2418. https://doi.org/10.1002/jcc.21533
Pham BQ, Gordon MS (2020) Development of the FMO/RI-MP2 fully analytic gradient using a hybrid-distributed/shared memory programming model. J Chem Theory Comput 16:1039–1054. https://doi.org/10.1021/acs.jctc.9b01082
Floris FM, Tomasi J, Ahuir JP (1991) Dispersion and repulsion contributions to the solvation energy: refinements to a simple computational model in the continuum approximation. J Comput Chem 12:784–791. https://doi.org/10.1002/jcc.540120703
Si D, Li H (2009) Heterogeneous conductor like solvation model. J Chem Phys 131:044123. https://doi.org/10.1063/1.3187527
Pierotti RA (1976) A scaled particle theory of aqueous and nonaqueous solutions. Chem Rev 76:717–726
Langlet J, Claverie P, Caillet J, Pullman A (1988) Improvements of the continuum model. 1. Application to the calculation of the vaporization thermodynamic quantities of nonassociated liquids. J. Phys. Chem. 92:1617–1163. https://doi.org/10.1021/j100317a048
Mori H, Ueno-Noto K, Osanai Y, Noro T, Fujiwara T, Klobukowski M, Miyoshi E (2009) Revised model core potentials for third-row transition–metal atoms from Lu to Hg. Chem. Phys. Lett. 476:317–322. https://doi.org/10.1016/j.cplett.2009.06.019
Barca GMJ, Bertoni C, Carrington L, Datta D, De Silva N, Deustua JE, Fedorov DG, Gour JR, Gunina AO, Guidez E, Harville T, Irle S, Ivanic J, Kowalski K, Leang SS, Li H, Li W, Lutz JJ, Magoulas I, Mato J, Mironov V, Nakata H, Pham BQ, Piecuch P, Poole D, Pruitt SR, Rendell AP, Roskop LB, Ruedenberg K, Sattasathuchana T, Schmidt MW, Shen J, Slipchenko L, Sosonkina M, Sundriyal V, Tiwari A, Vallejo JLG, Westheimer B, Włoch M, Xu P, Zahariev F, Gordon MS (2020) Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 152:154102. https://doi.org/10.1063/5.0005188
Okiyama Y, Nakano T, Watanabe C, Fukuzawa K, Mochizuki Y, Tanaka S (2018) Fragment Molecular Orbital Calculations with Implicit Solvent Based on the Poisson−Boltzmann Equation: Implementation and DNA Study. J Phys Chem B 122:4457–4471. https://doi.org/10.1021/acs.jpcb.8b01172
Fedorov DG (2019) Solvent screening in zwitterions analyzed with the fragment molecular orbital method. J. Chem. Theory Comput. 15:5404–5416. https://doi.org/10.1021/acs.jctc.9b00715
Fedorov DG, Asada N, Nakanishi I, Kitaura K (2014) The use of many-body expansions and geometry optimizations in fragment-based methods. Acc Chem Res 47:2846–2856. https://doi.org/10.1021/ar500224r
Yurenko YP, Novotný J, Sklenář V, Marek R (2014) Exploring non-covalent interactions in guanineand xanthine-based model DNA quadruplex structures: a comprehensive quantum chemical approach. Phys Chem Chem Phys 16:2072–2084. https://doi.org/10.1039/C3CP53875C
Fedorov DG (2020) Three-body energy decomposition analysis based on the fragment molecular orbital method. J. Phys. Chem. A 124:4956–4971. https://doi.org/10.1021/acs.jpca.0c03085
Zaccaria F, Paragi G, Fonseca Guerra C (2016) The role of alkali metal cations in the stabilization of guanine quadruplexes: why K+ is the best. Phys Chem Chem Phys 18:20895–20904. https://doi.org/10.1039/C6CP01030J
Mecozzi S, West AP, Dougherty DA (1996) Cation-π interactions in simple aromatics: electrostatics provide a predictive tool. J. Am. Chem. Soc. 118:2307–2308. https://doi.org/10.1021/ja9539608
Tateishi-Karimata H, Ohyama T, Muraoka T, Podbevsek P, Wawro AM, Tanaka S, Nakano S, Kinbara K, Plavec J, Sugimoto N (2017) Newly characterized interaction stabilizes DNA structure: oligoethylene glycols stabilize G-quadruplexes CH–π interactions. Nucleic Acids Res. 45:7021–7030. https://doi.org/10.1093/nar/gkx299
Meier-Menches SM, Aikman B, Döllerer D, Klooster WT, Coles SJ, Santi N, Luk L, Casini A, Bonsignore R (2020) Comparative biological evaluation and G-quadruplex interaction studies of two new families of organometallic gold(I) complexes featuring N-heterocyclic carbene and alkynyl ligands. J Inorg Biochem 202(110844):1–12. https://doi.org/10.1016/j.jinorgbio.2019.110844
Sen S, Perrin MW, Sedgwick AC, Lynch VM, Sessler JL, Arambula JF (2021) Covalent and non-covalent albumin binding of Au(I) bis-NHCs via post-synthetic amide modification. Chem Sci 12:7547–7553. https://doi.org/10.1039/D1SC01055G
Acknowledgements
We are thankful to Loriano Storchi of the Department of Pharmacy for computer support.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: RP; Formal analysis and investigation: RP; Writing—original draft preparation: RP; Writing—review and editing: CC, AM, NR, RP; Supervision: NR. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paciotti, R., Coletti, C., Marrone, A. et al. The FMO2 analysis of the ligand-receptor binding energy: the Biscarbene-Gold(I)/DNA G-Quadruplex case study. J Comput Aided Mol Des 36, 851–866 (2022). https://doi.org/10.1007/s10822-022-00484-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-022-00484-z