Abstract
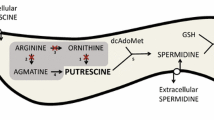
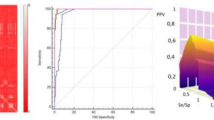
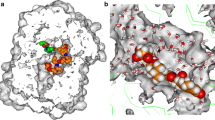
Chagas disease, also known as American trypanosomiasis, is a neglected tropical disease caused by the protozoa Trypanosoma cruzi, affecting nearly 7 million people only in the Americas. Polyamines are essential compounds for parasite growth, survival, and differentiation. However, because trypanosomatids are auxotrophic for polyamines, they must be obtained from the host by specific transporters. In this investigation, an ensemble of QSAR classifiers able to identify polyamine analogs with trypanocidal activity was developed. Then, a multi-template homology model of the dimeric polyamine transporter of T. cruzi, TcPAT12, was created with Rosetta, and then refined by enhanced sampling molecular dynamics simulations. Using representative snapshots extracted from the trajectory, a docking model able to discriminate between active and inactive compounds was developed and validated. Both models were applied in a parallel virtual screening campaign to repurpose known drugs as anti-trypanosomal compounds inhibiting polyamine transport in T. cruzi. Montelukast, Quinestrol, Danazol, and Dutasteride were selected for in vitro testing, and all of them inhibited putrescine uptake in biochemical assays, confirming the predictive ability of the computational models. Furthermore, all the confirmed hits proved to inhibit epimastigote proliferation, and Quinestrol and Danazol were able to inhibit, in the low micromolar range, the viability of trypomastigotes and the intracellular growth of amastigotes.
Graphical abstract










Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
PAHO (2019) Guidelines for the diagnosis and treatment of Chagas disease. https://www.who.int/publications-detail-redirect/9789275120439. Accessed 24 Jun 2022
Malone CJ, Nevis I, Fernández E, Sanchez A (2021) A Rapid Review on the efficacy and safety of pharmacological treatments for Chagas Disease. Trop Med Infect Dis 6:128. https://doi.org/10.3390/tropicalmed6030128
Pérez-Molina JA, Crespillo-Andújar C, Bosch-Nicolau P, Molina I (2020) Trypanocidal treatment of Chagas disease. Enfermedades Infecc Microbiol Clin Engl Ed. https://doi.org/10.1016/j.eimc.2020.04.011
Carrillo C, Canepa GE, Algranati ID, Pereira CA (2006) Molecular and functional characterization of a spermidine transporter (TcPAT12) from Trypanosoma cruzi. Biochem Biophys Res Commun 344:936–940. https://doi.org/10.1016/j.bbrc.2006.03.215
Carrillo C, Cejas S, Huber A et al (2003) Lack of arginine decarboxylase in Trypanosoma cruzi epimastigotes. J Eukaryot Microbiol 50:312–316. https://doi.org/10.1111/j.1550-7408.2003.tb00141.x
Michael AJ (2016) Polyamines in eukaryotes, bacteria, and archaea. J Biol Chem 291:14896–14903. https://doi.org/10.1074/jbc.R116.734780
Talevi A, Carrillo C, Comini M (2019) The thiol-polyamine metabolism of Trypanosoma cruzi: molecular targets and drug repurposing strategies. Curr Med Chem 26:6614–6635. https://doi.org/10.2174/0929867325666180926151059
Carrillo C, Cejas S, González NS, Algranati ID (1999) Trypanosoma cruzi epimastigotes lack ornithine decarboxylase but can express a foreign gene encoding this enzyme. FEBS Lett 454:192–196. https://doi.org/10.1016/s0014-5793(99)00804-2
Alberca LN, Sbaraglini ML, Morales JF et al (2018) Cascade ligand- and structure-based virtual screening to identify new trypanocidal compounds inhibiting putrescine uptake. Front Cell Infect Microbiol 8:173. https://doi.org/10.3389/fcimb.2018.00173
Soysa R, Venselaar H, Poston J et al (2013) Structural model of a putrescine-cadaverine permease from Trypanosoma cruzi predicts residues vital for transport and ligand binding. Biochem J 452:423–432. https://doi.org/10.1042/BJ20130350
Dietrich RC, Alberca LN, Ruiz MD et al (2018) Identification of cisapride as new inhibitor of putrescine uptake in Trypanosoma cruzi by combined ligand- and structure-based virtual screening. Eur J Med Chem 149:22–29. https://doi.org/10.1016/j.ejmech.2018.02.006
Reigada C, Valera-Vera EA, Sayé M et al (2017) Trypanocidal effect of isotretinoin through the inhibition of polyamine and amino acid transporters in Trypanosoma cruzi. PLoS Negl Trop Dis 11:e0005472. https://doi.org/10.1371/journal.pntd.0005472
Kowalczyk L, Ratera M, Paladino A et al (2011) Molecular basis of substrate-induced permeation by an amino acid antiporter. Proc Natl Acad Sci 108:3935–3940. https://doi.org/10.1073/pnas.1018081108
Song Y, DiMaio F, Wang RY-R et al (2013) High-resolution comparative modeling with RosettaCM. Structure 21:1735–1742. https://doi.org/10.1016/j.str.2013.08.005
Remmert M, Biegert A, Hauser A, Söding J (2012) HHblits: lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat Methods 9:173–175. https://doi.org/10.1038/nmeth.1818
Larsson P, Wallner B, Lindahl E, Elofsson A (2008) Using multiple templates to improve quality of homology models in automated homology modeling. Protein Sci Publ Protein Soc 17:990–1002. https://doi.org/10.1110/ps.073344908
Meier A, Söding J (2015) Automatic prediction of protein 3D structures by probabilistic multi-template homology modeling. PLoS Comput Biol. https://doi.org/10.1371/journal.pcbi.1004343
Case DA, Cheatham TE III, Darden T et al (2005) The amber biomolecular simulation programs. J Comput Chem 26:1668–1688. https://doi.org/10.1002/jcc.20290
Salomon-Ferrer R, Case DA, Walker RC (2013) An overview of the amber biomolecular simulation package. WIREs Comput Mol Sci 3:198–210. https://doi.org/10.1002/wcms.1121
Tian C, Kasavajhala K, Belfon KAA et al (2020) ff19SB: amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J Chem Theory Comput 16:528–552. https://doi.org/10.1021/acs.jctc.9b00591
Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA (2004) PDB2PQR: an automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res 32:W665–W667. https://doi.org/10.1093/nar/gkh381
Jo S, Kim T, Iyer VG, Im W (2008) CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem 29:1859–1865. https://doi.org/10.1002/jcc.20945
Wu EL, Cheng X, Jo S et al (2014) CHARMM-GUI membrane Builder toward realistic biological membrane simulations. J Comput Chem 35:1997–2004. https://doi.org/10.1002/jcc.23702
Hopkins CW, Le Grand S, Walker RC, Roitberg AE (2015) Long-time-step molecular dynamics through hydrogen mass repartitioning. J Chem Theory Comput 11:1864–1874. https://doi.org/10.1021/ct5010406
Alberca LN, Sbaraglini ML, Balcazar D et al (2016) Discovery of novel polyamine analogs with anti-protozoal activity by computer guided drug repositioning. J Comput Aided Mol Des 30:305–321. https://doi.org/10.1007/s10822-016-9903-6
Díaz MV, Miranda MR, Campos-Estrada C et al (2014) Pentamidine exerts in vitro and in vivo anti Trypanosoma cruzi activity and inhibits the polyamine transport in Trypanosoma cruzi. Acta Trop 134:1–9. https://doi.org/10.1016/j.actatropica.2014.02.012
Reigada C, Sayé M, Phanstiel O et al (2019) Identification of Trypanosoma cruzi polyamine transport inhibitors by computational drug repurposing. Front Med 6:256. https://doi.org/10.3389/fmed.2019.00256
Reigada C, Phanstiel O, Miranda MR, Pereira CA (2018) Targeting polyamine transport in Trypanosoma cruzi. Eur J Med Chem 147:1–6. https://doi.org/10.1016/j.ejmech.2018.01.083
Hasne MP, Coppens I, Soysa R, Ullman B (2010) A high-affinity putrescine-cadaverine transporter from Trypanosoma cruzi. Mol Microbiol 76:78–91. https://doi.org/10.1111/j.1365-2958.2010.07081.x
Alhossary A, Handoko SD, Mu Y, Kwoh C-K (2015) Fast, accurate, and reliable molecular docking with QuickVina 2. Bioinformatics 31:2214–2216. https://doi.org/10.1093/bioinformatics/btv082
Santos-Martins D, Solis-Vasquez L, Tillack AF, Sanner MF, Koch A, Forli S (2021) Accelerating AutoDock4 with GPUs and gradient-based local search. J Chem Theory Comput 17(2):1060–1073. https://doi.org/10.1021/acs.jctc.0c01006
Pettersen EF, Goddard TD, Huang CC et al (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Koes DR, Baumgartner MP, Camacho CJ (2013) Lessons learned in empirical scoring with smina from the CSAR 2011 Benchmarking Exercise. J Chem Inf Model 53:1893–1904. https://doi.org/10.1021/ci300604z
Fawcett T (2006) An introduction to ROC analysis. Pattern Recognit Lett 27:861–874. https://doi.org/10.1016/j.patrec.2005.10.010
Wang C, Zhang Y (2017) Improving scoring-docking-screening powers of protein-ligand scoring functions using random forest. J Comput Chem 38:169–177. https://doi.org/10.1002/jcc.24667
Birkholtz L-M, Williams M, Niemand J et al (2011) Polyamine homoeostasis as a drug target in pathogenic protozoa: peculiarities and possibilities. Biochem J 438:229–244. https://doi.org/10.1042/BJ20110362
Borges MN, Messeder JC, Figueroa-Villar JD (2004) Synthesis, anti-trypanosoma cruzi activity and micelle interaction studies of bisguanylhydrazones analogous to pentamidine. Eur J Med Chem 39:925–929. https://doi.org/10.1016/j.ejmech.2004.07.001
Braga SFP, Alves ÉVP, Ferreira RS et al (2014) Synthesis and evaluation of the antiparasitic activity of bis-(arylmethylidene) cycloalkanones. Eur J Med Chem 71:282–289. https://doi.org/10.1016/j.ejmech.2013.11.011
da Silva CF, da Silva PB, Batista MM et al (2010) The biological in vitro effect and selectivity of aromatic dicationic compounds on Trypanosoma cruzi. Mem Inst Oswaldo Cruz 105:239–245. https://doi.org/10.1590/s0074-02762010000300001
da Silva CF, Batista MM, da Batista D et al (2008) In vitro and in vivo studies of the trypanocidal activity of a diarylthiophene diamidine against Trypanosoma cruzi. Antimicrob Agents Chemother 52:3307–3314. https://doi.org/10.1128/AAC.00038-08
Daliry A, Pires MQ, Silva CF et al (2011) The Trypanocidal activity of Amidine Compounds does not correlate with their binding Affinity to Trypanosoma cruzi kinetoplast DNA▿. Antimicrob Agents Chemother 55:4765–4773. https://doi.org/10.1128/AAC.00229-11
Daliry A, Da Silva PB, Da Silva CF et al (2009) In vitro analyses of the effect of aromatic diamidines upon Trypanosoma cruzi. J Antimicrob Chemother 64:747–750. https://doi.org/10.1093/jac/dkp290
De Souza EM, da Silva PB, Nefertiti ASG et al (2011) Trypanocidal activity and selectivity in vitro of aromatic amidine compounds upon bloodstream and intracellular forms of Trypanosoma cruzi. Exp Parasitol 127:429–435. https://doi.org/10.1016/j.exppara.2010.10.010
González J (2007) Synthesis and antiparasitic evaluation of bis-2,5-[4-guanidinophenyl]thiophenes. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2006.11.006
Klenke B, Stewart M, Barrett MP et al (2001) Synthesis and biological evaluation of s-Triazine substituted polyamines as potential new anti-trypanosomal drugs. J Med Chem 44:3440–3452. https://doi.org/10.1021/jm010854+
Liew LPP, Kaiser M, Copp BR (2013) Discovery and preliminary structure-activity relationship analysis of 1,14-sperminediphenylacetamides as potent and selective antimalarial lead compounds. Bioorg Med Chem Lett 23:452–454. https://doi.org/10.1016/j.bmcl.2012.11.072
Lizzi F, Veronesi G, Belluti F et al (2012) Conjugation of quinones with natural polyamines: toward an expanded antitrypanosomatid profile. J Med Chem 55:10490–10500. https://doi.org/10.1021/jm301112z
Majumder S, Kierszenbaum F (1993) Inhibition of host cell invasion and intracellular replication of Trypanosoma cruzi by N,N’-bis(benzyl)-substituted polyamine analogs. Antimicrob Agents Chemother 37:2235–2238. https://doi.org/10.1128/AAC.37.10.2235
Menezes D, Valentim C, Oliveira MF, Vannier-Santos MA (2006) Putrescine analogue cytotoxicity against Trypanosoma cruzi. Parasitol Res 98:99–105. https://doi.org/10.1007/s00436-005-0010-1
Pacheco MG, de O CF, de Souza EM et al (2009) Trypanosoma cruzi: activity of heterocyclic cationic molecules in vitro. Exp Parasitol 123:73–80. https://doi.org/10.1016/j.exppara.2009.06.004
Patrick DA, Ismail MA, Arafa RK et al (2013) Synthesis and antiprotozoal activity of dicationic m-terphenyl and 1,3-dipyridylbenzene derivatives. J Med Chem 56:5473–5494. https://doi.org/10.1021/jm400508e
Silva CF, Batista MM, Mota RA et al (2007) Activity of “reversed” diamidines against Trypanosoma cruzi “in vitro.” Biochem Pharmacol 73:1939–1946. https://doi.org/10.1016/j.bcp.2007.03.020
Stephens CE, Brun R, Salem MM et al (2003) The activity of diguanidino and “reversed” diamidino 2,5-diarylfurans versus Trypanosoma cruzi and Leishmania donovani. Bioorg Med Chem Lett 13:2065–2069. https://doi.org/10.1016/s0960-894x(03)00319-6
Zhu X, Liu Q, Yang S et al (2012) Evaluation of arylimidamides DB1955 and DB1960 as candidates against visceral leishmaniasis and Chagas’ disease: in vivo efficacy, acute toxicity, pharmacokinetics, and toxicology studies. Antimicrob Agents Chemother 56:3690–3699. https://doi.org/10.1128/AAC.06404-11
Kawabata T (2011) Build-up algorithm for atomic correspondence between chemical structures. J Chem Inf Model 51:1775–1787. https://doi.org/10.1021/ci2001023
Sutton C, Sindelar M, McCallum A (2006) Reducing weight undertraining in structured discriminative learning. In: Proceedings of the main conference on human language technology conference of the North American chapter of the association of computational linguistics, pp 89–95. https://doi.org/10.3115/1220835.1220847
Toropova AP, Toropov AA (2017) CORAL: binary classifications (active/inactive) for drug-induced liver injury. Toxicol Lett 268:51–57. https://doi.org/10.1016/j.toxlet.2017.01.011
Konovalov DA, Llewellyn LE, Vander Heyden Y, Coomans D (2008) Robust cross-validation of Linear Regression QSAR Models. J Chem Inf Model 48:2081–2094. https://doi.org/10.1021/ci800209k
Rücker C, Rücker G, Meringer M (2007) y-Randomization and its variants in QSPR/QSAR. J Chem Inf Model 47:2345–2357. https://doi.org/10.1021/ci700157b
Wishart DS, Feunang YD, Guo AC et al (2018) DrugBank 5.0: a major update to the drugbank database for 2018. Nucleic Acids Res 46:D1074–D1082. https://doi.org/10.1093/nar/gkx1037
Tropsha A, Gramatica P, Gombar VK (2003) The importance of being Earnest: validation is the absolute essential for successful application and interpretation of QSPR Models. QSAR Comb Sci 22:69–77. https://doi.org/10.1002/qsar.200390007
Fraccaroli L, Ruiz MD, Perdomo VG et al (2022) Broadening the spectrum of ivermectin: Its effect on Trypanosoma cruzi and related trypanosomatids. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2022.885268
Miranda CG, Solana ME, Curto M, de los A et al (2015) A flow cytometer-based method to simultaneously assess activity and selectivity of compounds against the intracellular forms of Trypanosoma cruzi. Acta Trop 152:8–16. https://doi.org/10.1016/j.actatropica.2015.08.004
Miao Y, McCammon JA (2017) Chapter Six - Gaussian accelerated molecular dynamics: theory, implementation, and applications. In: Dixon DA (ed) Annual reports in computational chemistry. Elsevier, Amsterdam, pp 231–278
Wang J, Arantes PR, Bhattarai A et al (2021) Gaussian accelerated molecular dynamics: principles and applications. WIREs Comput Mol Sci. https://doi.org/10.1002/wcms.1521
Greene D, Qi R, Nguyen R et al (2019) Heterogeneous dielectric implicit membrane model for the calculation of MMPBSA binding free energies. J Chem Inf Model 59:3041–3056. https://doi.org/10.1021/acs.jcim.9b00363
Tan C, Tan Y-H, Luo R (2007) Implicit nonpolar solvent models. J Phys Chem B 111:12263–12274. https://doi.org/10.1021/jp073399n
Greene D, Botello-Smith WM, Follmer A et al (2016) Modeling membrane protein–ligand binding interactions: the human purinergic platelet receptor. J Phys Chem B 120:12293–12304. https://doi.org/10.1021/acs.jpcb.6b09535
The UniProt Consortium (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47:D506–D515. https://doi.org/10.1093/nar/gky1049
Ilgü H, Jeckelmann J-M, Gapsys V et al (2016) Insights into the molecular basis for substrate binding and specificity of the wild-type L-arginine/agmatine antiporter AdiC. Proc Natl Acad Sci 113:10358–10363. https://doi.org/10.1073/pnas.1605442113
Fang Y, Jayaram H, Shane T et al (2009) Structure of a prokaryotic virtual proton pump at 3.2 Å resolution. Nature 460:1040–1043. https://doi.org/10.1038/nature08201
Webb B, Sali A (2016) Comparative protein structure modeling using MODELLER. Curr Protoc Bioinform 54:5.6.1-5.6.37. https://doi.org/10.1002/cpbi.3
Chang J-M, Di Tommaso P, Taly J-F, Notredame C (2012) Accurate multiple sequence alignment of transmembrane proteins with PSI-Coffee. BMC Bioinformatics 13:S1. https://doi.org/10.1186/1471-2105-13-S4-S1
Dobson L, Reményi I, Tusnády GE (2015) CCTOP: a Consensus constrained TOPology prediction web server. Nucleic Acids Res 43:W408–W412. https://doi.org/10.1093/nar/gkv451
Ferri C, Hernández-Orallo J, Modroiu R (2009) An experimental comparison of performance measures for classification. Pattern Recognit Lett 30:27–38. https://doi.org/10.1016/j.patrec.2008.08.010
Markham A, Faulds D (1998) Montelukast Drugs 56:251–256. https://doi.org/10.2165/00003495-199856020-00010
Canavaci AMC, Sorgi CA, Martins VP et al (2014) The Acute Phase of Trypanosoma cruzi Infection Is Attenuated in 5-Lipoxygenase-Deficient Mice. In: Mediators Inflamm. https://www.hindawi.com/journals/mi/2014/893634/. Accessed 2 June 2020
Jaschevatzky OE, Anderman S, Shalit A et al (1979) The treatment of postmenopausal syndrome by monthly oral doses of quinestrol. Acta Obstet Gynecol Scand 58:175–178. https://doi.org/10.3109/00016347909154577
Pohlman GD, Pohlman EA, Crawford ED (2011) Dutasteride: a review of its use in the management of prostate disorders. Clin Med Insights Ther. https://doi.org/10.4137/CMT.S1956
Lindert S, Meiler J, McCammon JA (2013) Iterative molecular dynamics—rosetta protein structure refinement protocol to improve model quality. J Chem Theory Comput 9:3843–3847. https://doi.org/10.1021/ct400260c
Leelananda SP, Lindert S (2017) Iterative molecular dynamics–rosetta membrane protein structure refinement guided by cryo-EM densities. J Chem Theory Comput 13:5131–5145. https://doi.org/10.1021/acs.jctc.7b00464
Ludwiczak J, Jarmula A, Dunin-Horkawicz S (2018) Combining Rosetta with molecular dynamics (MD): a benchmark of the MD-based ensemble protein design. J Struct Biol 203:54–61. https://doi.org/10.1016/j.jsb.2018.02.004
Llanos MA, Sbaraglini ML, Villalba ML et al (2020) A structure-based approach towards the identification of novel antichagasic compounds: Trypanosoma cruzi carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 35:21–30. https://doi.org/10.1080/14756366.2019.1677638
Acknowledgements
L. Alberca, M. L. Sbaraglini, L. Fraccarolli, C. Alba Soto, C. Carrillo, L. Gavernet, and A. Talevi are members of Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (CONICET). M. A. Llanos, M. D. Ruiz, C. Miranda, and A. Pino-Martinez are fellowship holders of CONICET.
Funding
We thank Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (PICT 2017-0643 and PICT 2018-01124), Universidad Nacional de La Plata (Incentivos UNLP X785 and PPID program X043) and Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina PIP2015-0733.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by MAL, LNA, MDR, MLS, CM, APM and LF under the supervision of LG, CC, CDAS, and AT. The first draft of the manuscript was written by LG, CC, CDAS, AT, and MAL and then all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Llanos, M.A., Alberca, L.N., Ruiz, M.D. et al. A combined ligand and target-based virtual screening strategy to repurpose drugs as putrescine uptake inhibitors with trypanocidal activity. J Comput Aided Mol Des 37, 75–90 (2023). https://doi.org/10.1007/s10822-022-00491-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-022-00491-0