Abstract
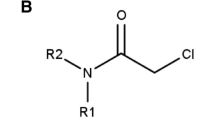
Covalent inhibition offers many advantages over non-covalent inhibition, but covalent warhead reactivity must be carefully balanced to maintain potency while avoiding unwanted side effects. While warhead reactivities are commonly measured with assays, a computational model to predict warhead reactivities could be useful for several aspects of the covalent inhibitor design process. Studies have shown correlations between covalent warhead reactivities and quantum mechanic (QM) properties that describe important aspects of the covalent reaction mechanism. However, the models from these studies are often linear regression equations and can have limitations associated with their usage. Applications of machine learning (ML) models to predict covalent warhead reactivities with QM descriptors are not extensively seen in the literature. This study uses QM descriptors, calculated at different levels of theory, to train ML models to predict reactivities of covalent acrylamide warheads. The QM/ML models are compared with linear regression models built upon the same QM descriptors and with ML models trained on structure-based features like Morgan fingerprints and RDKit descriptors. Experiments show that the QM/ML models outperform the linear regression models and the structure-based ML models, and literature test sets demonstrate the power of the QM/ML models to predict reactivities of unseen acrylamide warhead scaffolds. Ultimately, these QM/ML models are effective, computationally feasible tools that can expedite the design of new covalent inhibitors.










Similar content being viewed by others
Data availability
Structures of the warhead-containing, covalent compounds from the Novartis GSH assay data set (used to build the QM/ML models) are proprietary and cannot be disclosed. Literature test set structures can be found with their original sources and have also been compiled for convenience in the SI files. 2D structures were generated from SMILES strings using RDKit (http://www.rdkit.org). MoKa, which was used for protonation state generation, can be acquired from https://www.moldiscovery.com. Openeye Omega, which was used to generate conformers, can be acquired from http://www.eyesopen.com. The open source QM software package xTB is available at https://github.com/grimme-lab/xtb. The QM software package g09 can be acquired from https://gaussian.com. The QM software package Turbomole can be acquired from https://www.turbomole.org. The open source workflow that combines RDKit and QM calculations is available at https://github.com/ppqm/ppqm. Open source ML modeling packages included scikit-learn (https://github.com/scikit-learn/scikit-learn), PyTorch (https://pytorch.org/), and XGBoost (https://xgboost.readthedocs.io/en/stable/index.html). Hyperparameter tuning was performed with the open source software Hyperopt, which is available at http://hyperopt.github.io/hyperopt/.
Code availability
Not applicable.
References
Smith AJT, Zhang X, Leach AG, Houk KN (2008) Beyond picomolar affinities: quantitative aspects of noncovalent and covalent binding of drugs to proteins. J Med Chem 52(2):225–233. https://doi.org/10.1021/jm800498e
Yu HS, Gao C, Lupyan D, Wu Y, Kimura T, Wu C et al (2019) Toward atomistic modeling of irreversible covalent inhibitor binding kinetics. J Chem Inf Model 59(9):3955–3967. https://doi.org/10.1021/acs.jcim.9b00268
Luo YL (2021) Mechanism-based and computational-driven covalent drug design. J Chem Inf Model 61(11):5307–5311. https://doi.org/10.1021/acs.jcim.1c01278
Potashman MH, Duggan ME (2009) Covalent modifiers: an orthogonal approach to drug design. J Med Chem 52(5):1231–1246. https://doi.org/10.1021/jm8008597
Singh J, Petter RC, Baillie TA, Whitty A (2011) The resurgence of covalent drugs. Nat Rev Drug Discov 10(4):307–317. https://doi.org/10.1038/nrd3410
Mah R, Thomas JR, Shafer CM (2014) Drug discovery considerations in the development of covalent inhibitors. Bioorg Med Chem Lett 24(1):33–39. https://doi.org/10.1016/j.bmcl.2013.10.003
Cesco SD, Kurian J, Dufresne C, Mittermaier AK, Moitessier N (2017) Covalent inhibitors design and discovery. Eur J Med Chem 138:96–114. https://doi.org/10.1016/j.ejmech.2017.06.019
Mukherjee H, Grimster NP (2018) Beyond cysteine: recent developments in the area of targeted covalent inhibition. Curr Opin Chem Biol 44:30–38. https://doi.org/10.1016/j.cbpa.2018.05.011
Lonsdale R, Ward RA (2018) Structure-based design of targeted covalent inhibitors. Chem Soc Rev 47(11):3816–3830. https://doi.org/10.1039/c7cs00220c
Péczka N, Orgován Z, Ábrányi-Balogh P, Keserű GM (2022) Electrophilic warheads in covalent drug discovery: an overview. Expert Opin Drug Discov 17(4):413–422. https://doi.org/10.1080/17460441.2022.2034783
Baillie TA (2020) Approaches to mitigate the risk of serious adverse reactions in covalent drug design. Expert Opin Drug Discov 16(3):275–287. https://doi.org/10.1080/17460441.2021.1832079
Ábrányi-Balogh P, Petri L, Imre T, Szijj P, Scarpino A, Hrast M et al (2018) A road map for prioritizing warheads for cysteine targeting covalent inhibitors. Eur J Med Chem 160:94–107. https://doi.org/10.1016/j.ejmech.2018.10.010
Gehringer M, Laufer SA (2019) Emerging and re-emerging warheads for targeted covalent inhibitors: applications in medicinal chemistry and chemical biology. J Med Chem 62(12):5673–5724. https://doi.org/10.1021/acs.jmedchem.8b01153
Bianco G, Goodsell DS, Forli S (2020) Selective and effective: current progress in computational structure-based drug discovery of targeted covalent inhibitors. Trends Pharmacol Sci 41(12):1038–1049. https://doi.org/10.1016/j.tips.2020.10.005
Gehringer M (2020) Covalent inhibitors: back on track? Future Med Chem 12(15):1363–1368. https://doi.org/10.4155/fmc-2020-0118
Boike L, Henning NJ, Nomura DK (2022) Advances in covalent drug discovery. Nat Rev Drug Discov 21(12):881–898. https://doi.org/10.1038/s41573-022-00542-z
Krishnan S, Miller RM, Tian B, Mullins RD, Jacobson MP, Taunton J (2014) Design of reversible, cysteine-targeted Michael acceptors guided by kinetic and computational analysis. J Am Chem Soc 136(36):12624–12630. https://doi.org/10.1021/ja505194w
Birkholz A, Kopecky DJ, Volak LP, Bartberger MD, Chen Y, Tegley CM et al (2020) Systematic study of the glutathione reactivity of N-phenylacrylamides: 2. Effects of acrylamide substitution. J Med Chem 63(20):11602–11614. https://doi.org/10.1021/acs.jmedchem.0c00749
Awoonor-Williams E, Kennedy J, Rowley CN (2021) Measuring and predicting warhead and residue reactivity. The Design of Covalent-Based Inhibitors. Elsevier, New York, pp 203–227
Miseta A, Csutora P (2000) Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol 17(8):1232–1239. https://doi.org/10.1093/oxfordjournals.molbev.a026406
Zhang Y, Zhang D, Tian H, Jiao Y, Shi Z, Ran T et al (2016) Identification of covalent binding sites targeting cysteines based on computational approaches. Mol Pharm 13(9):3106–3118. https://doi.org/10.1021/acs.molpharmaceut.6b00302
Awoonor-Williams E, Rowley CN (2018) How reactive are druggable cysteines in protein kinases? J Chem Inf Model 58(9):1935–1946. https://doi.org/10.1021/acs.jcim.8b00454
Schwöbel JAH, Wondrousch D, Koleva YK, Madden JC, Cronin MTD, Schüürmann G (2010) Prediction of Michael-type acceptor reactivity toward glutathione. Chem Res Toxicol 23(10):1576–1585. https://doi.org/10.1021/tx100172x
Capoferri L, Lodola A, Rivara S, Mor M (2015) Quantum mechanics/molecular mechanics modeling of covalent addition between EGFR–cysteine 797 and N-(4-anilinoquinazolin-6-yl) acrylamide. J Chem Inf Model 55(3):589–599. https://doi.org/10.1021/ci500720e
Awoonor-Williams E, Rowley CN (2021) Modeling the binding and conformational energetics of a targeted covalent inhibitor to Bruton’s tyrosine kinase. J Chem Inf Model 61(10):5234–5242. https://doi.org/10.1021/acs.jcim.1c00897
Watt SKI, Charlebois JG, Rowley CN, Keillor JW (2022) A mechanistic study of thiol addition to N-phenylacrylamide. Org Biomol Chem 20(45):8898–8906. https://doi.org/10.1039/d2ob01369j
Watt SKI, Charlebois JG, Rowley CN, Keillor JW (2023) A mechanistic study of thiol addition to N-acryloylpiperidine. Org Biomol Chem 21(10):2204–2212. https://doi.org/10.1039/d2ob02223k
Keeley A, Petri L, Ábrányi-Balogh P, Keserű GM (2020) Covalent fragment libraries in drug discovery. Drug Discov Today 25(6):983–996. https://doi.org/10.1016/j.drudis.2020.03.016
Mihalovits LM, Ferenczy GG, Keserű GM (2021) The role of quantum chemistry in covalent inhibitor design. Int J Quantum Chem. https://doi.org/10.1002/qua.26768
Ertl P, Gerebtzoff G, Lewis R, Muenkler H, Schneider N, Sirockin F et al (2022) Chemical reactivity prediction: current methods and different application areas. Mol Inf 41(6):2100277. https://doi.org/10.1002/minf.202100277
Mihalovits LM, Ferenczy GG, Keserű GM (2020) Affinity and selectivity assessment of covalent inhibitors by free energy calculations. J Chem Inf Model 60(12):6579–6594. https://doi.org/10.1021/acs.jcim.0c00834
Bonatto V, Shamim A, dos R Rocho F, Leitão A, Luque FJ, Lameira J et al (2021) Predicting the relative binding affinity for reversible covalent inhibitors by free energy perturbation calculations. J Chem Inf Model 61(9):4733–4744. https://doi.org/10.1021/acs.jcim.1c00515
Awoonor-Williams E (2022) Estimating the binding energetics of reversible covalent inhibitors of the SARS-CoV-2 main protease: an in silico study. Phys Chem Chem Phys 24(38):23391–23401. https://doi.org/10.1039/d2cp03080b
Ward RA, Anderton MJ, Ashton S, Bethel PA, Box M, Butterworth S et al (2013) Structure- and reactivity-based development of covalent inhibitors of the activating and gatekeeper mutant forms of the epidermal growth factor receptor (EGFR). J Med Chem 56(17):7025–7048. https://doi.org/10.1021/jm400822z
Palazzesi F, Grundl MA, Pautsch A, Weber A, Tautermann CS (2019) A fast ab initio predictor tool for covalent reactivity estimation of acrylamides. J Chem Inf Model 59(8):3565–3571. https://doi.org/10.1021/acs.jcim.9b00316
Hermann MR, Pautsch A, Grundl MA, Weber A, Tautermann CS (2020) Covalent inhibitor reactivity prediction by the electrophilicity index—in and out of scope. J Comput-Aided Mol Des. 35(4):531–539. https://doi.org/10.1007/s10822-020-00342-w
Flanagan ME, Abramite JA, Anderson DP, Aulabaugh A, Dahal UP, Gilbert AM et al (2014) Chemical and computational methods for the characterization of covalent reactive groups for the prospective design of irreversible inhibitors. J Med Chem 57(23):10072–10079. https://doi.org/10.1021/jm501412a
Palazzesi F, Hermann MR, Grundl MA, Pautsch A, Seeliger D, Tautermann CS et al (2020) BIreactive: a machine-learning model to estimate covalent warhead reactivity. J Chem Inf Model 60(6):2915–2923. https://doi.org/10.1021/acs.jcim.9b01058
Hermann MR, Tautermann CS, Sieger P, Grundl MA, Weber A (2023) BIreactive: expanding the scope of reactivity predictions to propynamides. Pharmaceuticals 16(1):116. https://doi.org/10.3390/ph16010116
Oballa RM, Truchon JF, Bayly CI, Chauret N, Day S, Crane S et al (2007) A generally applicable method for assessing the electrophilicity and reactivity of diverse nitrile-containing compounds. Bioorg Med Chem Lett 17(4):998–1002. https://doi.org/10.1016/j.bmcl.2006.11.044
Shokhen M, Traube T, Vijayakumar S, Hirsch M, Uritsky N, Albeck A (2011) Differentiating serine and cysteine protease mechanisms by new covalent QSAR descriptors. ChemBioChem 12(7):1023–1026. https://doi.org/10.1002/cbic.201000459
Voice A, Tresadern G, van Vlijmen H, Mulholland A (2019) Limitations of ligand-only approaches for predicting the reactivity of covalent inhibitors. J Chem Inf Model 59(10):4220–4227. https://doi.org/10.1021/acs.jcim.9b00404
Liu R, Vázquez-Montelongo EA, Ma S, Shen J (2023) Quantum descriptors for predicting and understanding the structure-activity relationships of Michael acceptor warheads. J Chem Inf Model 63(15):4912–4923. https://doi.org/10.1021/acs.jcim.3c00720
Lonsdale R, Burgess J, Colclough N, Davies NL, Lenz EM, Orton AL et al (2017) Expanding the armory: predicting and tuning covalent warhead reactivity. J Chem Inf Model 57(12):3124–3137. https://doi.org/10.1021/acs.jcim.7b00553
Smith JM, Rowley CN (2015) Automated computational screening of the thiol reactivity of substituted alkenes. J Comput-Aided Mol Des 29(8):725–735. https://doi.org/10.1007/s10822-015-9857-0
Mulliken RS (1955) Electronic population analysis on LCAO–MO molecular wave functions. Int J Chem Phys 23(10):1833–1840. https://doi.org/10.1063/1.1740588
Hehre WJ (1977) Theory and practice of MO calculations on organic molecules. J Mol Struct 41(1):163. https://doi.org/10.1016/0022-2860(77)80052-5
Bannwarth C, Caldeweyher E, Ehlert S, Hansen A, Pracht P, Seibert J et al (2020) Extended tight-binding quantum chemistry methods. Wiley Interdiscip Rev. https://doi.org/10.1002/wcms.1493
MJ Frisch, GW Trucks, HB Schlegel, GE Scuseria, MA Robb, JR Cheeseman, G Scalmani, V Barone, GA Petersson, H Nakatsuji, X Li, M Caricato, AV Marenich, J Bloino, BG Janesko, R Gomperts, B Mennucci, HP Hratchian, JV Ortiz, AF Izmaylov, JL Sonnenberg, D Williams-Young, F Ding, F Lipparini, F Egidi, J Goings, B Peng, A Petrone, T Henderson, D Ranasinghe, VG Zakrzewski, J Gao, N Rega, G Zheng, W Liang, M Hada, M Ehara, K Toyota, R Fukuda, J Hasegawa, M Ishida, T Nakajima, Y Honda, O Kitao, H Nakai, T Vreven, K Throssell, Montgomery, Jr , JE Peralta, F Ogliaro, MJ Bearpark, JJ Heyd, EN Brothers, KN Kudin, VN Staroverov, TA Keith, R Kobayashi, J Normand, K Raghavachari, AP Rendell, JC Burant, SS Iyengar, J Tomasi, M Cossi, JM Millam, M Klene, C Adamo, R Cammi, JW Ochterski, RL Martin, K Morokuma, O Farkas, JB Foresman, DJ Fox.: Gaussian09 revision D.01. Gaussian Inc., Wallingford
TURBOMOLE V7 2 2017: a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH since 2007. http://www.turbomole.com
Udvarhelyi A, Rodde S, Wilcken R (2020) ReSCoSS: a flexible quantum chemistry workflow identifying relevant solution conformers of drug-like molecules. J Comput-Aided Mol Des 35(4):399–415. https://doi.org/10.1007/s10822-020-00337-7
RDKit. Open-source cheminformatics. http://www.rdkit.org
Molecular Discovery. MoKa, Borehamwood, UK. https://www.moldiscovery.com
OpenEye. OMEGA 4.2. 2.0., Cadence molecular sciences, Santa Fe, NM. http://www.eyesopen.com
Bannwarth C, Ehlert S, Grimme S (2019) GFN2-xTB—an accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J Chem Theory Comput 15(3):1652–1671. https://doi.org/10.1021/acs.jctc.8b01176
Ehlert S, Stahn M, Spicher S, Grimme S (2021) Robust and efficient implicit solvation model for fast semiempirical methods. J Chem Theory Comput 17(7):4250–4261. https://doi.org/10.1021/acs.jctc.1c00471
Pye CC, Ziegler T, van Lenthe E, Louwen JN (2009) An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package—-part II. COSMO for real solvents. Can J Chem 87(7):790–797. https://doi.org/10.1139/v09-008
Louwen JN, Pye CC, van Lenthe E, Austin ND, McGarrity ES, Xiong R et al (2023) AMS 2023.1 COSMO-RS. SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. http://www.scm.com
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652. https://doi.org/10.1063/1.464913
Tirado-Rives J, Jorgensen WL (2008) Performance of B3LYP density functional methods for a large set of organic molecules. J Chem Theory Comput 4(2):297–306. https://doi.org/10.1021/ct700248k
Smith JM, Alahmadi YJ, Rowley CN (2013) Range-separated DFT functionals are necessary to model Thio-Michael additions. J Chem Theory Comput 9(11):4860–4865. https://doi.org/10.1021/ct400773k
Awoonor-Williams E, Walsh AG, Rowley CN (2017) Modeling covalent-modifier drugs. Biochim Biophys Acta 1865(11):1664–1675. https://doi.org/10.1016/j.bbapap.2017.05.009
Awoonor-Williams E, Isley WC, Dale SG, Johnson ER, Yu H, Becke AD et al (2019) Quantum chemical methods for modeling covalent modification of biological thiols. J Comput Chem 41(5):427–438. https://doi.org/10.1002/jcc.26064
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10(44):6615. https://doi.org/10.1039/b810189b
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O et al (2011) Scikit-learn: machine learning in python. J Mach Learn Res 12:2825–2830
Paszke A, Gross S, Massa F, Lerer A, Bradbury J, Chanan G et al (2019) PyTorch: an imperative style, high-performance deep learning library. Advances in neural information processing systems, vol 32. Curran Associates Inc, New York, pp 8024–8035
Chen T, Guestrin C (2016) XGBoost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining (KDD ’16), pp 785–794, New York. https://doi.org/10.1145/2939672.2939785
Heid E, Greenman KP, Chung Y, Li SC, Graff DE, Vermeire FH et al (2023) Chemprop: machine learning package for chemical property prediction. ChemRxiv. https://doi.org/10.26434/chemrxiv-2023-3zcfl
Bergstra J, Yamins D, Cox D (2013) Making a science of model search: hyperparameter optimization in hundreds of dimensions for vision architectures. In: Dasgupta S, McAllester D, editors. In: Proceedings of the 30th international conference on machine learning, vol 28 of proceedings of machine learning research, Atlanta, GA: PMLR, pp 115–123. https://proceedings.mlr.press/v28/bergstra13.html
Morgan HL (1965) The generation of a unique machine description for chemical structures—a technique developed at chemical abstracts service. J Chem Doc 5(2):107–113. https://doi.org/10.1021/c160017a018
Breiman L (2001) Random forests. Mach Learn 45(1):5–32. https://doi.org/10.1023/a:1010933404324
Cee VJ, Volak LP, Chen Y, Bartberger MD, Tegley C, Arvedson T et al (2015) Systematic study of the glutathione (GSH) reactivity of N-arylacrylamides: 1. Effects of aryl substitution. J Med Chem 58(23):9171–9178. https://doi.org/10.1021/acs.jmedchem.5b01018
Acknowledgements
The authors thank Ernest Awoonor-Williams and Rainer Wilcken for helpful discussions, Jimmy Kroman for assistance with the conformer generation/QM calculation workflow [73], and Andreas Lingel, Daniel Gosling, and Damien Hubert for their work with the GSH assay.
Funding
Partial funding support was provided by Anne Granger and the Innovation Postdoctoral Fellowship Program at Novartis Biomedical Research (formerly NIBR).
Author information
Authors and Affiliations
Contributions
Study conception and design were performed by Viktor Hornak, Callum J. Dickson, Aaron D. Danilack, and Jose S. Duca. GSH assay data collection and analysis were performed by Stephane Rodde and colleagues. QM calculations were performed by Aaron D. Danilack with assistance from Hagen Munkler. ML model construction was led by Cihan Soylu and Mike Fortunato. The first draft of the manuscript was written by Aaron D. Danilack, and all authors provided feedback and edits. The final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare, financial or otherwise, related to the completion of this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Danilack, A.D., Dickson, C.J., Soylu, C. et al. Reactivities of acrylamide warheads toward cysteine targets: a QM/ML approach to covalent inhibitor design. J Comput Aided Mol Des 38, 21 (2024). https://doi.org/10.1007/s10822-024-00560-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10822-024-00560-6