Abstract
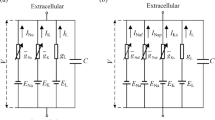
Electrical stimulation of the central nervous system creates both orthodromically propagating action potentials, by stimulation of local cells and passing axons, and antidromically propagating action potentials, by stimulation of presynaptic axons and terminals. Our aim was to understand how antidromic action potentials navigate through complex arborizations, such as those of thalamic and basal ganglia afferents—sites of electrical activation during deep brain stimulation. We developed computational models to study the propagation of antidromic action potentials past the bifurcation in branched axons. In both unmyelinated and myelinated branched axons, when the diameters of each axon branch remained under a specific threshold (set by the antidromic geometric ratio), antidromic propagation occurred robustly; action potentials traveled both antidromically into the primary segment as well as “re-orthodromically” into the terminal secondary segment. Propagation occurred across a broad range of stimulation frequencies, axon segment geometries, and concentrations of extracellular potassium, but was strongly dependent on the geometry of the node of Ranvier at the axonal bifurcation. Thus, antidromic activation of axon terminals can, through axon collaterals, lead to widespread activation or inhibition of targets remote from the site of stimulation. These effects should be included when interpreting the results of functional imaging or evoked potential studies on the mechanisms of action of DBS.








Similar content being viewed by others
Notes
Model code available at http://senselab.med.yale.edu/senselab/modeldb/ShowModel.asp?model=3810.
References
Anderson, T. R., Hu, B., Iremonger, K., & Kiss, Z. H. (2006). Selective attenuation of afferent synaptic transmission as a mechanism of thalamic deep brain stimulation-induced tremor arrest. Journal of Neuroscience, 26, 841–850.
Asanuma, K., Tang, C., Ma, Y., Dhawan, V., Mattis, P., Edwards, C., et al. (2006). Network modulation in the treatment of Parkinson’s disease. Brain, 129, 2667–2678.
Ashby, P., & Rothwell, J. C. (2000). Neurophsysiologic aspects of deep brain stimulation. Neurology, 55(Suppl 6), S17–S20.
Ashby, P., Paradiso, G., Saint-Cyr, J. A., Chen, R., Lang, A. E., & Lozano, A. M. (2001). Potentials recorded at the scalp by stimulation near the human subthalamic nucleus. Clinical Neurophysiology, 112, 431–437.
Baker, K. B., Montgomery, E. B. Jr., Rezai, A. R., Burgess, R., & Luders, H. O. (2002). Subthalamic nucleus deep brain stimulus evoked potentials: Physiological and therapeutic implications. Movement Disorders, 17, 969–983.
Baldissera, F., Lundberg, A., & Udo, M. (1972) Stimulation of pre- and postsynaptic elements in the red nucleus. Experimental Brain Research, 15, 151–167.
Barron, D. H., & Matthews, B. H. C. (1935). Intermittent conduction in the spinal cord. Journal of Physiology (London), 85, 73–103.
Benazzouz, A., Piallat, B., Pollak, P., & Benabid, A. L. (1995). Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: Electrophysiological data. Neuroscience Letter, 189, 77–80.
Biedenbach, M. A., De Vito, J. L., & Brown, A. C. (1986). Pyramidal tract of the cat: Axon size and morphology. Experimental Brain Research, 61(2), 303–310.
Butovas, S., & Schwarz, C. (2003). Spatiotemporal effects of microstimulation in rat neocortex: A parametric study using multielectrode recordings. Journal of Neurophysiology, 90, 3024–3039.
Ceballos-Baumann, A. O., Boecker, H., Bartenstein, P., von Falkenhayn, I., Riescher, H., Conrad, B., et al. (1999). A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: Enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Archives of Neurology, 56(8), 997–1003.
Chung, S. H., Raymond, S. A., & Lettivin, J. Y. (1970). Multiple meaning in single visual units. Brain, Behavior and Evolution, 3, 72–101.
Coleman, G. T., Mahns, D. A., Zhang, H. Q., & Rowe, M. J. (2003). Impulse propagation over tactile and kinaesthetic sensory axons to central target neurons of the cunaeate nucleus in the cat. Journal of Physiology, 550, 553–562.
Deibner, M. P., Pollack, P., Passingham, R., et al. (1993). Thalamic stimulation and suppression of tremor: Evidence of a cerebellar deactivation using PET. Brain, 116, 267–279.
Deschenes, M., & Landry, P. (1980). Axonal branch diameter and spacing of nodes in the terminal arborization of identified thalamic and cortical neurons. Brain Research, 191(2), 538–544.
Dostrovsky, J. O., & Lozano, A. M. (2002). Mechanisms of deep brain stimulation. Movement Disorders, 17(Suppl. 3), S63–S68.
Dostrovsky, J. O., Levy, R., Wu, J. P., Hutchison, W. D., Tasker, R. R., & Lozano, A. M. (2000). Microstimulation-induced inhibition of neuronal firing in human globus pallidus. Journal of Neurophysiology, 84, 570–574.
Engel, D. A., & Jonas, P. M. (2004). Presynaptic voltage-gated Na+ channels boost action potentials in hippocampal mossy fiber boutons. Program No. 397.4. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience.
Filali, M., Hutchison, W. D., Palter, V. N., Lozano, A. M., & Dostrovsky, J. O. (2004). Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Experimental Brain Research, 156, 274–281.
Frankenhaeuser, B., & Hodgkin, A. L. (1956). The after-effects of impulses in the giant nerve fibres of Loligo. Journal of Physiology (London), 131, 341–376.
Gauthier, J., Parent, M., Levesque, M., & Parent, A. (1999). The axonal arborization of single nigrostriatal neurons in rats. Brain Research, 834(1–2), 228–232.
Grafton, S. T., Turner, R. S., Desmurget, M., Bakay, R., Delong, M., Vitek, J., et al. (2006). Normalizing motor-related brain activity: Subthalamic nucleus stimulation in Parkinson disease. Neurology, 66(8), 1192–1199.
Goldstein, S. S., & Rall, W. (1974). Changes of action potential shape and velocity for changing core conductor geometry. Biophysical Journal, 14(10), 731–757.
Goldfinger, M. D. (1990). Random sequence stimulation of the G1 hair afferent unit. Somatosensory Motor Research, 7, 19–45.
Goldfinger, M. D. (2005). Highly efficient propagation of random impulse trains across unmyelinated axonal branch points: Modifications by periaxonal K+ accumulation and sodium channel kinetics. In Reeke GN, Poznanski RR, Lindsay KA, Rosenberg JR, Sporns O (eds.), Modeling in the Neurosciences, 2nd ed. (p. 479–530) Boca Raton, Florida: Taylor and Francis.
Grill, W. M., & McIntyre, C. C. (2001). Extracellular excitation of central neurons: Implications for the mechanism of deep brain stimulation. Thalamus & Related Systems, 1, 269–277.
Grill, W. M., Snyder, A. N., & Miocinovic, S. (2004). Deep brain stimulation creates an informational lesion of the stimulated nucleus. NeuroReport, 15(7), 1137–1140.
Grossman, Y., Parnas, I., & Spira, M. E. (1979). Differential conduction block in branches of a bifurcating axon. Journal of Physiology (London), 295, 283–305.
Gustafsson, B., & Jankowska, E. (1976). Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. Journal of Physiology, 258, 33–61.
Hanajima, R., Ashby, P., Lozano, A. M., Lang, A. E., & Chen, R. (2004). Single pulse stimulation of the human subthalamic nucleus facilitates the motor cortex at short intervals. Journal of Neurophysiology, 92, 1937–1943.
Haslinger, B., Kalteis, K., Boecker, H., Alesch, F., & Ceballos-Baumann, A. O. (2005). Frequency-correlated decreases of motor cortex activity associated with subthalamic nucleus stimulation in Parkinson’s disease. NeuroImage, 28, 598–606.
Hess, A., & Young, J. Z. (1949). Correlation of internodal length and fibre diameter in the central nervous system. Nature, 164, 490–491.
Hildebrand, C., Remahl, S., Persson, H., & Bjartmar, C. (1993). Myelinated nerve fibres in the CNS. Progress in Neurobiology, 40, 319–384.
Hines, M. L., & Carnevale, N. T. (2001). NEURON: A tool for neuroscientists. Neuroscientist, 7(2), 123–135.
Hoppe, D., Chvatal, A., Kettenmann, H., Orkand, R. K., & Ransom, B. R. (1991). Characteristics of activity-dependent potassium accumulation in mammalian peripheral nerve in vitro. Brain Research, 552, 106–112.
Hursch, J. B. (1939). Conduction velocity and diameter of nerve fibers. American Journal of Physiology, 127, 131–139.
Jankowska, E., Padel, Y., & Tanaka, R. (1975). The mode of activation of pyramidal tract cells by intracortical stimuli. Journal of Physiology, 249, 617–636.
Jech, R., Urgosik, D., Tintera, J., Nebuzelsky, A., Krasensky, J., Liscak, R., et al. (2001). Functional magnetic resonance imaging during deep brain stimulation: A pilot study in four patients with Parkinson’s disease. Movement Disorders, 16(4), 1126–1132.
Joyner, R. W., Westerfield, M., & Moore, J. W. (1980). Effects of cellular geometry on current flow during a propagated action potential. Biophysical Journal, 31(2), 183–194.
Kakei, S., N. A., J., & Shinoda, Y. (2001). Thalamic terminal morphology and distribution of single corticothalamic axons originating from layers 5 and 6 of the cat motor cortex. Journal of Comparative Neurology, 437(2), 170–185.
Khattab, F. I. (1968). Branching of the nodal axon in the cerebral cortex of mice. Brain Research, 9(1), 149–151.
Kitai, S. T., & Deniau, J. M. (1981). Cortical inputs to the subthalamus: Intracellular analysis. Brain Research, 214(2), 411–415.
Kultas-Ilinsky, K., Sivan-Loukianova, E., & Ilinsky, L. A. (2003). Reevaluation of the primary motor cortex connections with the thalamus in primates. Journal of Comparative Neurology, 457(2), 133–158.
Levesque, M., & Parent, A. (2005). The striatofugal fiber system in primates: A reevaluation of its organization based on single-axon tracing studies. Proceedings of the National Academy of Sciences, 102, 11888–11893.
Lieberman, A. R., Webster, K. E., & Spacek, J. (1972). Multiple myelinated branches from nodes of Ranvier in the central nervous system. Brain Research, 44(2), 652–655.
Manor, Y., Koch, C., & Segev, I. (1991). Effect of geometrical irregularities on propagation delay in axonal trees. Biophysical Journal, 60(6), 1434–1437.
McCreery, D. B., & Agnew, W. F. (1983). Changes in extracellular potassium and calcium concentration and neural activity during prolonged electrical stimulation of the cat cerebral cortex at defined charge densities. Experimental Neurology, 79, 371–396.
McIntyre, C. C., Richardson, A. G., & Grill, W. M. (2002). Modeling the excitability of mammalian nerve fibers: Influence of afterpotentials on the recovery cycle. Journal of Neurophysiology, 87, 995–1006.
McIntyre, C. C., Grill, W. M., Sherman, D. L., & Thakor, N. V. (2004). Cellular effects of deep brain stimulation: Model-based analysis of activation and inhibition. Journal of Neurophysiology, 91, 1457–1469.
Meeks, J. P., Jiang, X., & Mennerick, S. (2005). Action potential fidelity during normal and epileptiform activity in paired soma-axon recordings from rat hippocampus. Journal of Physiology, 566(2), 425–441.
Montgomery, E. B. (2006). Effects of GPi stimulation on human thalamic neuronal activity. Clinical Neurophysiology, 117(12), 2691–2702.
Mulloney, B., & Selverston, A. (1972). Antidromic action potentials fail to demonstrate known interactions between neurons. Science, 177, 69–72.
Parent, M., & Parent, A. (2002). Axon collateralization in primate basal ganglia and related thalamic nuclei. Thalamus & Related Systems, 2, 71–86.
Parent, M., & Parent, A. (2004). The pallidofugal motor fiber system in primates. Parkinsonism & Related Disorders, 10, 203–211.
Parent, M., Levesque, M., & Parent, A. (1999). The pallidofugal projection system in primates: evidence for neurons branching ipsilaterally and contralaterally to the thalamus and brainstem. Journal of Chemical Neuroanatomy, 16, 153–165.
Parnas, I. (1972). Differential block at high frequency at branches of a single axon innervating two muscles. Journal of Neurophysiology, 35, 903–914.
Parnas, I., & Segev, I. (1974). A mathematical model for conduction of action potentials along bifurcating axons. Journal of Physiology, 295, 323–343.
Perlmutter, J. S., Mink, J. W., Bastian, A. J., Zackowski, K., Hershey, T., Miyawaki. E., et al. (2002). Blood flow responses to deep brain stimulation of thalamus. Neurology, 58, 1388–1394.
Pittman, Q. J. (1983). Increases in antidromic latency of neurohypophyseal neurons during sustained activation. Neuroscience Letter, 37, 239–243.
Ranck, J. B. Jr. (1975). Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Research, 98, 417–440.
Rattay, F., & Aberham, M. (1993). Modeling axon membranes for functional electrical stimulation. IEEE Transactions on Biomedical Engieering, 40, 1201–1209.
Rubinstein, J. T. (1993). Axon termination conditions for electrical stimulation. IEEE Transactions on Biomedical Engineering, 40, 654–663.
Saito, K. (1979). Branchings at the central node of Ranvier, observed in the anterior horn and Clarke’s nucleus of the cat. An electron microscopic study. Neuroscience, 4(3), 391–399.
Sato, F., Lavallee, P., Levesque, M., & Parent, A. (2000a) Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. Journal of Comparative Neurology, 417, 17–31.
Sato, F., Parent, M., Levesque, M., & Parent, A. (2000b) Axonal branching pattern of neurons of the subthalamic nucleus in primates. Journal of Comparative Neurology, 424, 142–152.
Shefi, O., Herl, A., Chklovskii, D. B., Ben-Jacob, E., & Ayali, A. (2004). Biophysical constraints on neuronal branching. Neurocomputing, 58–60, 487–495.
Spacek, J. (2000). Node of Ranvier. Atlas of Ultrastructural Neurocytology. Available at: http://synapses.mcg.edu/atlas/2_3_1_5.stm. Accessed on July 15, 2005.
Starr, P. A., Vitek, J. L., & Bakay, R. A. E. (1998). Deep brain stimulation for movement disorders. Neurosurgery Clinics of North America, 9, 381–402.
Tauc, L., & Hughes, G. M. (1964). Modes of initiation and propagation of spikes in the branching axons of molluscan central nervous system. Journal of General Physiology, 46, 533–549.
Tolias, A. S., Sultan, F., Augath, M., Oeltermann, A., Tehovnik, E. J., Schiller, P. H., et al. (2005). Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron, 48, 901–911.
Trost, M., Su, S., Su, P., Yen, R. F., Tseng, H. M., Barnes, A., et al. (2006). Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease. NeuroImage, 31(1), 301–307.
Waxman, S. G., Kocsis, J. D., & Stys, P. K. (1995). The Axon. New York: Oxford University Press.
Welter, M. L., Houeto, J. L., Bonnet, A. M., Bejjani, P. B., Mesnage, V., Dormont, D., et al. (2004). Effects of high-frequency stimulation on subthalamic neuronal activity in parkinsonian patients. Archives of Neurology, 61, 89–96.
Westerfield, M., Joyner, R. W., & Moore, J. W. (1978). Temperature-sensitive conduction failure at axon branch points. Journal of Neurophysiology, 41, 1–8.
Wu, Y. R., Levy, R., Ashby, P., Tasker, R. R., & Dostrovsky, J. O. (2001). Does stimulation of the GPi control dyskinesia by activating inhibitory axons? Movement Disordorders, 16, 208–216.
Zhou, L., & Chiu, S. Y. (2001). Computer model for action potential propagation through branch point in myelinated nerves. Journal of Neurophysiology, 85(1), 197–210.
Acknowledgements
This work was supported by grant R01 NS40894 from the National Institutes of Health. The authors thank Dr. Alan Dorval for critically reading the original manuscript before submission.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grant R01 NS40894 from the National Institutes of Health.
Action Editor: David Terman
Rights and permissions
About this article
Cite this article
Grill, W.M., Cantrell, M.B. & Robertson, M.S. Antidromic propagation of action potentials in branched axons: implications for the mechanisms of action of deep brain stimulation. J Comput Neurosci 24, 81–93 (2008). https://doi.org/10.1007/s10827-007-0043-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-007-0043-9