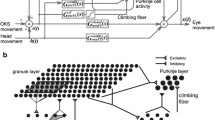
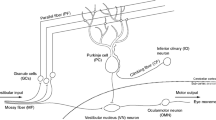
Abstract
There is a debate regarding whether motor memory is stored in the cerebellar cortex, or the cerebellar nuclei, or both. Memory may be acquired in the cortex and then be transferred to the cerebellar nuclei. Based on a dynamical system modeling with a minimal set of variables, we theoretically investigated possible mechanisms of memory transfer and consolidation in the context of vestibulo-ocular reflex learning. We tested different plasticity rules for synapses in the cerebellar nuclei and took robustness of behavior against parameter variation as the criterion of plausibility of a model variant. In the most plausible scenarios, mossy-fiber nucleus-neuron synapses or Purkinje-cell nucleus-neuron synapses are plastic on a slow time scale and store permanent memory, whose content is passed from the cerebellar cortex storing transient memory. In these scenarios, synaptic strengths are potentiated when the mossy-fiber afferents to the nuclei are active during a pause in Purkinje-cell activities. Furthermore, assuming that mossy fibers create a limited variety of signals compared to parallel fibers, our model shows partial memory transfer from the cortex to the nuclei.







Similar content being viewed by others
References
Aizenman, C. D., & Linden, D. J. (1999). Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. Journal of Neurophysiology, 82, 1697–1709.
Aizenman, C. D., & Linden, D. J. (2000). Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nature Neuroscience, 3, 109–111.
Aizenman, C. D., Manis, P. B., & Linden, D. J. (1998). Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron, 21, 827–835.
Akemann, W., & Knöpfel, T. (2006). Interaction of Kv3 potassium channels and resurgent sodium current influences the rate of spontaneous firing of Purkinje neurons. Journal of Neuroscience, 26, 4602–4612.
Albus, J. S. (1971). A theory of cerebellar function. Mathematical Biosciences, 10, 25–61.
Armstrong, D. M., & Rawson, J. A. (1979). Activity patterns of cerebellar cortical neurons and climbing fibre afferents in the awake cat. Journal of Physiology, 289, 425–448.
Boyden, E. S., Katoh, A., & Raymond, J. L. (2004). Cerebellum-dependent learning: The role of multiple plasticity mechanisms. Annual Review of Neuroscience, 27, 581–609.
Coesmans, M., Weber, J. T., De Zeeuw, C. I., & Hansel, C. (2004). Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron, 44, 691–700.
Dayan, P., & Abbott, L. F. (2001). Theoretical neuroscience—computational and mathematical modeling of neural systems. MIT.
Davies, P., & Melvill Jones, G. (1976). An adaptive neural model compatible with plastic changes induced in the human vestibulo-ocular reflex by prolonged optical reversal of vision. Brain Research, 103, 546–550.
De Schutter, E., & Bjaalie, J. G. (2001). Coding in the granular layer of the cerebellum. Progress in Brain Research, 130, 279–296.
du Lac, S., Raymond, J. L., Sejnowski, T. J., & Lisberger, S. G. (1995). Learning and memory in the vestibulo-ocular reflex. Annual Review of Neuroscience, 18, 409–441.
Fujita, M. (1982). Adaptive filter model of the cerebellum. Biological Cybernetics, 45, 195–206.
Goldberg, J. M., & Fernandez, C. (1971). Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. Journal of Neurophysiology, 34, 635–660.
Hansel, C., Linden, D. J., & D’Angelo, E. (2001). Beyond parallel fiber LTD: the diversity of synaptic and nonsynaptic plasticity in the cerebellum. Nature Neuroscience, 4, 467–475.
Ito, M. (2001). Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiological Reviews, 81, 1143–1195.
Ito, M., Jastreboff, P. J., & Miyashita, Y. (1982a). Specific effects of unilateral lesions in the flocculus upon eye movements in albino rabbits. Experimental Brain Research, 45, 233–242.
Ito, M., Sakurai, M., & Tongroach, P. (1982b). Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. Journal of Physiology, 324, 113–134.
Kassardjian, C. D., Yao-Fang, T., Chung, J. Y. J., Heskin, R., Peterson, M. J., & Broussard, D. M. (2005). The site of a motor memory shifts with consolidation. Journal Neuroscience, 25, 7979–7985.
Kleim, J. A., Freeman Jr., J. H., Bruneau, R., Nolan, B. C., Cooper, N. R., Zook, A. et al. (2002). Synapse formation is associated with memory storage in the cerebellum. Proceedings of the National Academy of Sciences of the United States of America, 99, 13228–13231.
Kramer, P. D., Shelhamer, M., & Zee, D. S. (1995). Short-term adaptation of the phase of the vestibulo-ocular reflex (VOR) in normal human subjects. Experimental Brain Research, 106, 318–326.
LeDoux, M. S., Hurst, D. C., & Lorden, J. F. (1998). Single-unit activity of cerebellar nuclear cells in the awake genetically dystonic rat. Neuroscience, 86, 533–545.
Lisberger, S. G. (1988). The neural basis for learning of simple motor skills. Science, 242, 728–735.
Lisberger, S. F., & Sejnowski, T. J. (1992). Motor learning in a recurrent network model based on the vestibulo-ocular reflex. Nature, 360, 159–161.
Luebke, A. E., & Robinson, D. A. (1994). Gain changes of the cat's vestibulo-ocular reflex after flocculus deactivation. Experimental Brain Research, 98, 379–390.
Marr, D. (1969). A theory of cerebellar cortex. Journal of Physiology, 202, 437–470.
Masuda, N., & Amari, S. (2006). Modeling memory transfer and savings in cerebellar motor learning. Advances in Neural Information Processing Systems, 18, 859–866 (Y. Weiss, B. Scholkopf, J. Platt Eds.).
Mauk, M. D. (1997). Roles of cerebellar cortex and nuclei in motor learning: Contradictions or clues? Neuron, 18, 343–346.
Mauk, M. D., & Donegan, N. H. (1997). A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learning & Memory, 3, 130–158.
McClelland, J. L., McNaughton, B. L., & O'Reilly, R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102, 419–457.
Medina, J. F., Garcia, K. S., & Mauk, M. D. (2001). A mechanism for savings in the cerebellum. Journal of Neuroscience, 21, 4081–4089.
Medina, J. F., Garcia, K. S., Nores, W. L., Taylor, N. M., & Mauk, M. D. (2000). Timing mechanisms in the cerebellum: Testing predictions of a large-scale computer simulation. Journal of Neuroscience, 20, 5516–5525.
Medina, J. F., & Mauk, M. D. (1999). Simulations of cerebellar motor learning: Computational analysis of plasticity at the mossy fiber to deep nucleus synapse. Journal of Neuroscience, 19, 7140–7151.
Medina, J. F., Repa, J. C., Mauk, M. D., & LeDoux, J. E. (2002). Parallels between cerebellum- and amygdala-dependent conditioning. Nature Review Neuroscience, 3, 122–131.
Miles, F. A., & Lisberger, S. G. (1981). Plasticity in the vestibulo-ocular reflex: A new hypothesis. Annual Review Neuroscience, 4, 273–299.
Miyachi, S., Hikosaka, O., & Lu, X. (2002). Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Experimental Brain Research, 146, 122–126.
Miyachi, S., Hikosaka, O., Miyashita, K., Kárádi, Z., & Rand, M. K. (1997). Differential roles of monkey striatum in learning of sequential hand movement. Experimental Brain Research, 115, 1–5.
Morishita, W., & Sastry, B. R. (1996). Postsynaptic mechanisms underlying long-term depression of GABAergic transmission in neurons of the deep cerebellar nuclei. Journal of Neurophysiology, 76, 59–68.
Muellbacher, W., Ziemann, U., Wissel, J., Dang, N., Kofler, M., Facchini, S. et al. (2002). Early consolidation in human primary motor cortex. Nature, 415, 640–644.
Nagao, S., Kitamura, T., Nakamura, N., Hiramatsu, T., & Yamada, J. (1997). Differences of the primate flocculus and ventral paraflocculus in the mossy and climbing fiber input organization. Journal of Comparative Neurology, 382, 480–498.
Nagao, S., & Kitazawa, H. (2003). Effects of reversible shutdown of the monkey flocculus on the retention of adaptation of the horizontal vestibulo-ocular reflex. Neuroscience, 118, 563–570.
Osanai, R., Nagao, S., Kitamura, T., Kawabata, I., & Yamada, J. (1999). Differences in mossy and climbing afferent sources between flocculus and ventral and dorsal paraflocculus in the rat. Experimental Brain Research, 124, 248–264.
Ouardouz, M., & Sastry, B. R. (2000). Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. Journal of Neurophysiology, 84, 1414–1421.
Palkovits, M., Mezey, É., Hámori, J., & Szentágothai, J. (1977). Quantitative histological analysis of the cerebellar nuclei in the cat. I. numerical data on cells and on synapses. Experimental Brain Research, 28, 189–209.
Pasupathy, A., & Miller, E. K. (2005). Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature, 433, 873–876.
Penhune, V. P., & Doyon, J. (2002). Dynamic cortical and subcortical networks in learning and delayed recall of timed motor sequences. Journal of Neuroscience, 22, 1397–1406.
Penhune, V. B., & Doyon, J. (2005). Cerebellum and M1 interaction during early learning of timed motor sequences. Neuroimage, 26, 801–812.
Perrett, S. P., Luis, B. P., & Mauk, M. D. (1993). Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. Journal of Neuroscience, 13, 1708–1718.
Perrett, S. P., & Mauk, M. D. (1995). Extinction of conditioned eyelid responses requires the anterior lobe of cerebellar cortex. Journal of Neuroscience, 15, 2074–2080.
Peterson, B. W., Baker, J. F., & Houk, J. C. (1991). A model of adaptive control of vestibuloocular reflex based on properties of cross-axis adaptation. Annual New York Academy Science, 627, 319–337.
Pugh, J. R., & Raman, I. M. (2006). Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron, 51, 113–123.
Racine, R. J., Wilson, D. A., Gingell, R., & Sunderland, D. (1986). Long-term potentiation in the interpositus and vestibular nuclei in the rat. Experimental Brain Research, 63, 158–162.
Raymond, J. L., Lisberger, S. G., & Mauk, M. D. (1996). The cerebellum: A neuronal learning machine? Science, 272, 1126–1131.
Repa, J. C., Muller, J., Apergis, J., Desrochers, T. M., Zhou, Y., & LeDoux, J. E. (2001). Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nature Neuroscience, 4, 724–731.
Roland, N. C., & Jaeger, D. (2005). Coding of tactile response properties in the rat deep cerebellar nuclei. Journal of Neurophysiology, 94, 1236–1251.
Raymond, J. L., & Lisberger, S. G. (1998). Neural learning rules for the vestibulo-ocular reflex. Journal of Neuroscience, 18, 9112–9129.
Sakurai, M. (1987). Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. Journal of Physiology, 394, 463–480.
Shutoh, F., Ohki, M., Kitazawa, H., Itohara, S., & Nagao, S. (2006). Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience, 139, 767–777.
Stickgold, R., Hobson, J. A., Fosse, R., & Fosse, M. (2001). Sleep, learning, and dreams: Off-line memory reprocessing. Science, 294, 1052–1057.
Sugihara, I., Ebata, S., & Shinoda, Y. (2004). Functional compartmentalization in the flocculus and the ventral dentate and dorsal group y nuclei: an analysis of single olivocerebellar axonal morphology. Journal of Compensation and Benefits, 470, 113.
Thach, W. T. (1968). Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. Journal of Neurophysiology, 31, 785–797.
Walker, M. P., Brakefield, T., Hobson, J. A., & Stickgold, R. (2003). Dissociable stages of human memory consolidation and reconsolidation. Nature, 425, 616–620.
Yamazaki, T., & Tanaka, S. (2005). Neural modeling of an internal clock. Neural Computation, 17, 1032–1058.
Zhang, W., & Linden, D. J. (2006). Long-term depression at the mossy fiber-deep cerebellar nucleus synapse. Journal of Neuroscience, 26, 6935–6944.
Acknowledgments
We thank S. Nagao, T. Knöpfel, and C. Rockland for helpful discussions and carefully reading the manuscript. This work is supported by Special Postdoctoral Researchers Program of RIKEN.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Erik De Schutter
Appendices
Appendix A: Fast–slow analysis with static MF and PF firing and MF–VN plasticity
When the vestibular signal is static, we set ω=0, θ= π/2, and hence set the vestibular signal sin(ωt + θ)=1. Given that the MF and PF firing rates are static with f(a)=1, we have <sin(ωt + θ)u> i =<sin(ωt + θ)x> i =<u> i =<x> i = <ux t> i,j =<xu t> j,i =<xx t> i,j =1. Here, <u> i , for example, is time average of the i-th MF signal. Because the PC–VN synapse is assumed to be static under MF–VN plasticity, we set b = 1. Let us assume m = n = 1 (and quit bold notations) and perform fast–slow analysis. Similar analysis works for general m and n.
In an early stage of learning, the PF–PC synapse w evolves much faster than the MF–VN synapse v does. The fast nullcline defined by setting dw/dt = 0 in Eq. (8) is given by
On a short timescale, w and v converge onto this line.
1.1 A.1 CF-driven MF–VN plasticity
For the CF-driven learning, the slow nullcline to which w and v converge in a long run is given by setting dv/dt = 0 in Eq. (11):
A trajectory of the synaptic weights in the w–v plane approaches somewhere on the fast nullcline (Eq. (27)) and then slides along it toward the equilibrium obtained as the crossing of the two nullclines. The crossing is given by
The error at the equilibrium is
which is small given η 1 ≫ η 3.
1.2 A.2 Hebbian MF–VN plasticity
For the Hebbian learning, the w-nullcline is given by Eq. (27), and the v-nullcline derived from Eq. (13) becomes
The equilibrium is given by
Because the relative magnitudes of η 1 η 6 and η 3 η 4 are indecisive, the sign of η 1 η 6 − η 3 η 4 + η 3 η 6 is indefinite.
1.3 A.3 PC-driven MF–VN plasticity
For the PC-driven learning, the slow nullcline derived from Eq. (15) becomes
and the equilibrium is given by
Appendix B: Fast–slow analysis for PC-driven PC–VN plasticity
For PC-driven PC–VN plasticity, we can analytically obtain the equilibrium solutions. The initial synaptic weights are w = w 0, v = v 0, and b = b 0, which are chosen to yield the initial gain R 0. This condition together with Eqs. (16) and (18) provides the relations:
Because we assumed η 1 ≫ η 3 and η 4 ≫ η 6, there are two equilibria (w*, b*) given by
and
In both solutions, the final error is
which is small. We discard the second solution because it is unstable.
Appendix C: Gain and phase learning with dynamic neural responses and MF–VN plasticity
With f(a) = sin(a), we obtain <x> = <u> = 0, <sin(ωt + θ)x> i = cos(θ − φ i )/2, <xu t> i,j =<ux t> j,i =cos(ψ j − φ i )/2, and <xx t> i,j =cos(φ j − φ i )/2. The signals with closer phase leads are more correlated. Then, Eq. (8) reads
3.1 C.1 CF-driven MF–VN plasticity
With the CF-driven learning rule, Eq. (11) reads
In terms of the order parameters defined by
the equilibrium for the desired output R sin (ωt + θ) is obtained by solving
The phase leads φ i and ψ i are assumed to be distributed uniformly on [0,2π] and [−Δ ψ , Δ ψ ], respectively. Assuming that n and m are large, we have, for example,
For notational convenience, we write A c (Δ ψ ) = 1 + sin 2Δ ψ /2Δ ψ and A s (Δ ψ ) = 1 − sin 2Δ ψ /2Δ ψ . We note that 1 ≤ A c ≤ 2, and A s decreases in Δ ψ . Particularly, A s ≅ 2Δ ψ 2/3 becomes small as Δ ψ → 0. Then, the equilibrium is given by
where α = c or s. The amount of the memory stored in the MF–VN synapses is given by
where
If MFs create any delay elements (Δ ψ = π, A c (Δ ψ ) = A s (Δ ψ ) = 1), Eq. (52) results in θ D = θ, that is, the perfect phase learning by the MF–VN synapses. However, r D = η 3 η 4/(η 1 η 6 + η 3 η 4 + 4η 3 η 6) can be considerably smaller than the ideal value (= 1) because η 1 η 6 may be as large as η 3 η 4 in general. The MF–VN synapses learn the desired gain only for a restricted parameter range, and gain transfer is not robust against parameter variation. This is in line with the result of the gain-only theory.
Furthermore, the discrepancy between θ D and θ cannot be ignored when Δ ψ = O((η 1 η 6/η 3 η 4)1/2) i.e. when Δ ψ is of the order of (η 1 η 6/η 3 η 4)1/2. Because η 1 ≫ η 3 and η 4 ≫ η 6, one cannot tell without additional information whether η 1 η 6 ≫ η 3 η 4, η 1 η 6 ≅ η 3 η 4, or η 1 η 6 ≪ η 3 η 4. Because η 1 η 6/η 3 η 4 is not necessarily small, transfer can degrade even for a large Δ ψ .
3.2 C.2 Hebbian MF–VN plasticity
With the Hebbian rule, Eq. (13) reads
By solving the equilibrium in combination with Eq. (45), we have
which leads to
When MFs create any phase leads (Δ ψ = π), Eq. (57) implies perfect transfer of the target phase (θ D = θ). However, r D = η 1 η 4/(4η 1 η 6 + 16η 3 η 6 − 4η 3 η 4), derived from Eq. (56), is indefinite because 4η 1 η 6 + 16η 3 η 6 − 4η 3 η 4 can take an arbitrary value. Consequently, unrealistic phenomena such as overlearning (r D > 1) can arise in the model. Regarding phase learning, the error is prominent when Δ ψ is as small as Δ ψ = O((η 6/η 4)1/2) (recall η 4 ≫ η 6), which is suitable.
3.3 C.3 PC-driven MF–VN plasticity
With the PC-driven learning, Eq. (15) reads
We derive
which in combination with Eq. (50) yields r D (Eq. (23)) and θ D (Eq. (24)). The amount of the memory stored in the PF–PC synapses is represented by
Rights and permissions
About this article
Cite this article
Masuda, N., Amari, Si. A computational study of synaptic mechanisms of partial memory transfer in cerebellar vestibulo-ocular-reflex learning. J Comput Neurosci 24, 137–156 (2008). https://doi.org/10.1007/s10827-007-0045-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-007-0045-7