Abstract
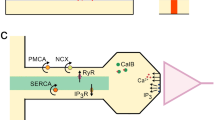
Dendritic spines are thought to compartmentalize second messengers like Ca2+. The notion of isolated spine signaling, however, was challenged by the recent finding that under certain conditions mobile endogenous Ca2+-binding proteins may break the spine limit and lead to activation of Ca2+-dependent dendritic signaling cascades. Since the size of spines is variable, the spine neck may be an important regulator of this spino-dendritic crosstalk. We tested this hypothesis by using an experimentally defined, kinetic computer model in which spines of Purkinje neurons were coupled to their parent dendrite by necks of variable geometry. We show that Ca2+ signaling and calmodulin activation in spines with long necks is essentially isolated from the dendrite, while stubby spines show a strong coupling with their dendrite, mediated particularly by calbindin D28k. We conclude that the spine neck geometry, in close interplay with mobile Ca2+-binding proteins, regulates the spino-dendritic crosstalk.






Similar content being viewed by others
References
Allbritton, N. L., Meyer, T., & Stryer, L. (1992). Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science, 258, 1812–1815. doi:10.1126/science.1465619.
Araya, R., Jiang, J., Eisenthal, K. B., & Yuste, R. (2006). The spine neck filters membrane potentials. Proceedings of the National Academy of Sciences of the United States of America, 103, 17961–17966. doi:10.1073/pnas.0608755103.
Augustine, G. J., Santamaria, F., & Tanaka, K. (2003). Local calcium signaling in neurons. Neuron, 40, 331–346. doi:10.1016/S0896-6273(03)00639-1.
Barski, J. J., Hartmann, J., Rose, C. R., Hoebeek, F., Mörl, K., Noll-Hussong, M., et al. (2003). Calbindin in cerebellar Purkinje cells is a critical determinant of the precision of motor coordination. The Journal of Neuroscience, 23, 3469–3477.
Berggård, T., Szczepankiewicz, O., Thulin, E., & Linse, S. (2002). Myo-inositol monophosphatase is an activated target of calbindin D28k. The Journal of Biological Chemistry, 277, 41954–41959. doi:10.1074/jbc.M203492200.
Bhalla, U. S. (2004a). Signaling in small subcellular volumes. I. Stochastic and diffusion effects on individual pathways. Biophysical Journal, 87, 733–744. doi:10.1529/biophysj.104.040469.
Bhalla, U. S. (2004b). Signaling in small subcellular volumes. II. Stochastic and diffusion effects on synaptic network properties. Biophysical Journal, 87, 745–753. doi:10.1529/biophysj.104.040501.
Blackwell, K. T. (2006). An efficient stochastic diffusion algorithm for modeling second messengers in dendrites and spines. Journal of Neuroscience Methods, 157, 142–153. doi:10.1016/j.jneumeth.2006.04.003.
Bloodgood, B. L., & Sabatini, B. L. (2005). Neuronal activity regulates diffusion across the neck of dendritic spines. Science, 310, 866–869. doi:10.1126/science.1114816.
Bonhoeffer, T., & Yuste, R. (2002). Spine motility. Phenomenology, mechanisms, and function. Neuron, 35, 1019–1027. doi:10.1016/S0896-6273(02)00906-6.
Crick, F. (1982). Do dendritic spines twitch? Trends in Neurosciences, 5, 44–46. doi:10.1016/0166-2236(82)90020-0.
Daniel, H., Levenes, C., & Crepél, F. (1998). Cellular mechanisms of cerebellar LTD. Trends in Neurosciences, 21, 401–407. doi:10.1016/S0166-2236(98)01304-6.
Denk, W., Sugimori, M., & Llinás, R. (1995). Two types of calcium responses limited to single spines in cerebellar Purkinje cells. Proceedings of the National Academy of Sciences of the United States of America, 92, 8279–8282. doi:10.1073/pnas.92.18.8279.
Dunaevsky, A., Tashiro, A., Majewska, A., Mason, C., & Yuste, R. (1999). Developmental regulation of spine motility in the mammalian central nervous system. Proceedings of the National Academy of Sciences of the United States of America, 96, 13438–13443. doi:10.1073/pnas.96.23.13438.
Eberhard, M., & Erne, P. (1991). Calcium binding to fluorescent calcium indicators : calcium green, calcium orange and calcium crimson. Biochemical and Biophysical Research Communications, 180, 209–215. doi:10.1016/S0006-291X(05)81278-1.
Eberhard, M., & Erne, P. (1994). Calcium and magnesium binding to rat parvalbumin. European Journal of Biochemistry, 222, 21–26. doi:10.1111/j.1432-1033.1994.tb18836.x.
Eilers, J., Augustine, G. J., & Konnerth, A. (1995). Subthreshold synaptic Ca2+ signalling in fine dendrites and spines of cerebellar Purkinje neurons. Nature, 373, 155–158. doi:10.1038/373155a0.
Eilers, J., Takechi, H., Finch, E. A., Augustine, G. J., & Konnerth, A. (1997). Local dendritic Ca2+ signaling induces cerebellar LTD. Learning & Memory (Cold Spring Harbor, N.Y.), 3, 159–168. doi:10.1101/lm.4.1.159.
Faas et al. (2004), Soc. Neurosci. Abstr. No 165.5.
Fiala, J. C., & Harris, K. M. (2005). Dendrite Structure. In G. Stuart, N. Spruston, & M. Häusser (Eds.), Dendrites (pp. 1–34). Oxford: Oxford University Press.
Fierro, L., & Llano, I. (1996). High endogenous calcium buffering in Purkinje cells from rat cerebellar slices. The Journal of Physiology, 496, 617–625.
Finch, E. A., & Augustine, G. J. (1998). Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature, 396, 753–756. doi:10.1038/25541.
Fischer, M., Kaech, S., Knutti, D., & Matus, A. (1998). Rapid actin-based plasticity in dendritic spines. Neuron, 20, 847–854. doi:10.1016/S0896-6273(00)80467-5.
Franks, K. M., & Sejnowski, T. J. (2002). Complexity of calcium signaling in synaptic spines. BioEssays, 24, 1130–1144. doi:10.1002/bies.10193.
Gamble, E., & Koch, C. (1987). The dynamics of free calcium in dendritic spines in response to repetitive synaptic input. Science, 236, 1131–1135. doi:10.1126/science.3495885.
Guthrie, P. B., Segal, M., & Kater, S. B. (1991). Independent regulation of calcium revealed by imaging dendritic spines. Nature, 354, 76–80. doi:10.1038/354076a0.
Harris, K. M., & Stevens, J. K. (1988). Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. The Journal of Neuroscience, 8, 4455–4469.
Hartmann, J., & Konnerth, A. (2005). Determinants of postsynaptic Ca2+ signaling in Purkinje neurons. Cell Calcium, 37, 459–466. doi:10.1016/j.ceca.2005.01.014.
Hayashi, Y., & Majewska, A. K. (2005). Dendritic spine geometry: Functional implication and regulation. Neuron, 46, 529–532. doi:10.1016/j.neuron.2005.05.006.
Heim, N., & Griesbeck, O. (2004). Genetically encoded indicators of cellular calcium dynamics based on troponin C and green fluorescent protein. The Journal of Biological Chemistry, 279, 14280–14286. doi:10.1074/jbc.M312751200.
Heim, N., Garaschuk, O., Friedrich, M. W., Mank, M., Milos, R. I., Kovalchuk, Y., et al. (2007). Improved calcium imaging in transgenic mice expressing a troponin C-based biosensor. Nature Methods, 4, 127–129. doi:10.1038/nmeth1009.
Helmchen, F., Imoto, K., & Sakmann, B. (1996). Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurones. Biophysical Journal, 70, 1069–1081. doi:10.1016/S0006-3495(96)79653-4.
Hernjak, N., Slepchenko, B. M., Fernald, K., Fink, C. C., Fortin, D., Moraru, I., et al. (2005). Modeling and analysis of calcium signaling events leading to long-term depression in cerebellar Purkinje cells. Biophysical Journal, 89, 3790–3806. doi:10.1529/biophysj.105.065771.
Holcman, D., Schuss, Z., & Korkotian, E. (2004). Calcium dynamics in dendritic spines and spine motility. Biophysical Journal, 87, 81–91. doi:10.1529/biophysj.103.035972.
Holthoff, K., Tsay, D., & Yuste, R. (2002). Calcium dynamics of spines depend on their dendritic location. Neuron, 33, 425–437. doi:10.1016/S0896-6273(02)00576-7.
Kaiser, K. M., Zilberter, Y., & Sakmann, B. (2001). Back-propagating action potentials mediate calcium signalling in dendrites of bitufted interneurons in layer 2/3 of rat somatosensory cortex. The Journal of Physiology, 535, 17–31. doi:10.1111/j.1469-7793.2001.t01-1-00017.x.
Keller, D. X., Franks, K. M., Bartol, T. M., & Sejnowski, T. J. (2008). Calmodulin activation by calcium transients in the postsynaptic density of dendritic spines. PLoS ONE, 3, E2045. doi:10.1371/journal.pone.0002045.
Koch, C., & Zador, A. (1993). The function of dendritic spines: devices subserving biochemical rather than electrical compartmentalization. The Journal of Neuroscience, 13, 413–422.
Korkotian, E., & Segal, M. (2001). Regulation of dendritic spine motility in cultured hippocampal neurons. The Journal of Neuroscience, 21, 6115–6124.
Kosaka, T., Kosaka, K., Nakayama, T., Hunziker, W., & Heizmann, C. W. (1993). Axons and axon terminals of cerebellar Purkinje cells and basket cells have higher levels of parvalbumin immunoreactivity than somata and dendrites: quantitative analysis by immunogold labeling. Experimental Brain Research, 93, 483–491. doi:10.1007/BF00229363.
Lee, S. - H., Schwaller, B., & Neher, E. (2000a). Kinetics of Ca2+ binding to parvalbumin in bovine chromaffin cells: Implications for [Ca2+] transients of neuronal dendrites. The Journal of Physiology, 525, 419–432. doi:10.1111/j.1469-7793.2000.t01-2-00419.x.
Lee, S. - H., Rosenmund, C., Schwaller, B., & Neher, E. (2000b). Differences in Ca2+ buffering properties between excitatory and inhibitory hippocampal neurons from the rat. The Journal of Physiology, 525, 405–418. doi:10.1111/j.1469-7793.2000.t01-3-00405.x.
Maeda, H., Ellis-Davies, G. C., Ito, K., Miyashita, Y., & Kasai, H. (1999). Supralinear Ca2+ signaling by cooperative and mobile Ca2+ buffering in Purkinje neurons. Neuron, 24, 989–1002. doi:10.1016/S0896-6273(00)81045-4.
Majewska, A., Tashiro, A., & Yuste, R. (2000a). Regulation of spine calcium dynamics by rapid spine motility. The Journal of Neuroscience, 20, 8262–8268.
Majewska, A., Brown, E., Ross, J., & Yuste, R. (2000b). Mechanisms of calcium decay kinetics in hippocampal spines: role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. The Journal of Neuroscience, 20, 1722–1734.
Markram, H., Helm, J., & Sakmann, B. (1995). Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. The Journal of Physiology, 485, 1–20.
Markram, H., Roth, A., & Helmchen, F. (1998). Competitive calcium binding: implications for dendritic calcium signaling. Journal of Computational Neuroscience, 5, 331–348. doi:10.1023/A:1008891229546.
Mayawala, K., Vlachos, D. G., & Edwards, J. S. (2006). Spatial modeling of dimerization reaction dynamics in the plasma membrane: Monte Carlo vs. continuum differential equations. Biophysical Chemistry, 121, 194–208. doi:10.1016/j.bpc.2006.01.008.
Müller, W., & Connor, J. A. (1991). Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature, 354, 73–76. doi:10.1038/354073a0.
Müller, A., Kukley, M., Stausberg, P., Beck, H., Müller, W., & Dietrich, D. (2005). Endogenous Ca2+ buffer concentration and Ca2+ microdomains in hippocampal neurons. The Journal of Neuroscience, 25, 558–565. doi:10.1523/JNEUROSCI.3799-04.2005.
Nägerl, U. V., Novo, D., Mody, I., & Vergara, J. L. (2000). Binding kinetics of calbindin-D28k determined by flash photolysis of caged Ca2+. Biophysical Journal, 79, 3009–3018. doi:10.1016/S0006-3495(00)76537-4.
Neher, E. (1999). Some quantitative aspects of calcium fluorimetry. In R. Yuste, F. Lenny, & A. Konnerth (Eds.), Imaging neurons: a laboratory manual (pp. 31.31–31.11). New York: Cold Spring Harbor Laboratory Press.
Neher, E., & Augustine, G. J. (1992). Calcium gradients and buffers in bovine chromaffin cells. The Journal of Physiology, 450, 273–301.
Nimchinsky, E. A., Sabatini, B. L., & Svoboda, K. (2002). Structure and function of dendritic spines. Annual Review of Physiology, 64, 313–353. doi:10.1146/annurev.physiol.64.081501.160008.
Noguchi, J., Matsuzaki, M., Ellis-Davies, G. C., & Kasai, H. (2005). Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron, 46, 609–622. doi:10.1016/j.neuron.2005.03.015.
Rao, C. V., Wolf, D. M., & Arkin, A. P. (2002). Control, exploitation and tolerance of intracellular noise. Nature, 420, 231–237. doi:10.1038/nature01258.
Rexhausen, U. (1992). Bestimmung der Diffusionseigenschaften von Fluoreszenzfarbstoffen in verzweigten Nervenzellen unter Verwendung eines rechnergesteuerten Bildverarbeitungssystems. University of Göttingen, Diploma thesis.
Sabatini, B. L., Oertner, T. G., & Svoboda, K. (2002). The life cycle of Ca2+ ions in dendritic spines. Neuron, 33, 439–452. doi:10.1016/S0896-6273(02)00573-1.
Santamaria, F., Wils, S., De Schutter, E., & Augustine, G. J. (2006). Anomalous diffusion in purkinje cell dendrites caused by spines. Neuron, 52(4), 635–648. doi:10.1016/j.neuron.2006.10.025.
Schmidt, H., Stiefel, K., Racay, P., Schwaller, B., & Eilers, J. (2003a). Mutational analysis of dendritic Ca2+ kinetics in rodent Purkinje cells: role of parvalbumin and calbindin D28k. The Journal of Physiology, 551, 13–32. doi:10.1113/jphysiol.2002.035824.
Schmidt, H., Brown, E. B., Schwaller, B., & Eilers, J. (2003b). Diffusional mobility of parvalbumin in spiny dendrites of cerebellar Purkinje neurons quantified by fluorescence recovery after photobleaching. Biophysical Journal, 84, 2599–2608. doi:10.1016/S0006-3495(03)75065-6.
Schmidt, H., Schwaller, B., & Eilers, J. (2005). Calbindin D28k targets myo-inositol monophosphatase in spines and dendrites of cerebellar Purkinje neurons. Proceedings of the National Academy of Sciences of the United States of America, 102, 5850–5855. doi:10.1073/pnas.0407855102.
Schmidt, H., Kunerth, S., Wilms, C., Strotmann, R., & Eilers, J. (2007). Spino-dendritic cross-talk in rodent Purkinje neurons mediated by endogenous Ca2+-binding proteins. The Journal of Physiology, 581, 619–629. doi:10.1113/jphysiol.2007.127860.
Sjöström, P. J., Rancz, E. A., Roth, A., & Häusser, M. (2008). Dendritic excitability and synaptic plasticity. Physiological Reviews, 88, 769–840. doi:10.1152/physrev.00016.2007.
Spacek, J., & Hartmann, M. (1983). Three-dimensional analysis of dendritic spines. I. Quantitative observations related to dendritic spine and synaptic morphology in cerebral and cerebellar cortices. Anatomy and Embryology, 167, 289–310. doi:10.1007/BF00298517.
Svoboda, K., Tank, D. W., & Denk, W. (1996). Direct measurement of coupling between dendritic spines and shafts. Science, 272, 716–719. doi:10.1126/science.272.5262.716.
Svoboda, K., Denk, W., Kleinfeld, D., & Tank, D. W. (1997). In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature, 385, 161–165. doi:10.1038/385161a0.
Takechi, H., Eilers, J., & Konnerth, A. (1998). A new class of synaptic responses involving calcium release in dendritic spines. Nature, 396, 757–760. doi:10.1038/25547.
Tsay, D., & Yuste, R. (2004). On the electrical function of dendritic spines. Trends in Neurosciences, 27, 77–83. doi:10.1016/j.tins.2003.11.008.
Vecellio, M., Schwaller, B., Meyer, M., Hunziker, W., & Celio, M. R. (2000). Alterations in Purkinje cell spines of calbindin D-28 k and parvalbumin knock-out mice. The European Journal of Neuroscience, 12, 945–954. doi:10.1046/j.1460-9568.2000.00986.x.
Volfovsky, N., Parnas, H., Segal, M., & Korkotian, E. (1999). Geometry of dendritic spines affects calcium dynamics in hippocampal neurons: Theory and experiments. Journal of Neurophysiology, 82, 450–462.
Wilms, C. D., Schmidt, H., & Eilers, J. (2006). Quantitative two-photon Ca2+ imaging via fluorescence lifetime analysis. Cell Calcium, 40, 73–79. doi:10.1016/j.ceca.2006.03.006.
Xia, Z., & Storm, D. R. (2005). The role of calmodulin as a signal integrator for synaptic plasticity. Nature Reviews. Neuroscience, 6, 267–276. doi:10.1038/nrn1647.
Yuste, R., & Denk, W. (1995). Dendritic spines as basic functional units of neuronal integration. Nature, 375, 682–684. doi:10.1038/375682a0.
Zador, A., Koch, C., & Brown, T. H. (1990). Biophysical model of a Hebbian synapse. Proceedings of the National Academy of Sciences of the United States of America, 87, 6718–6722. doi:10.1073/pnas.87.17.6718.
Acknowledgements
We thank Stefan Hallermann for critical discussion and Gudrun Bethge for technical assistance. The work was supported by grants from the Bundesministerium für Bildung und Forschung to J.E.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor:
Erik Deschutter
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Spino-dendritic coupling during rapid Ca2+ signaling for stubby and slim spines (left and right column, respectively). Same simulation as in Fig. 3 (thick lines) but with the addition of simulations for the upper and lower bound of Ca2+ signals, i.e. for the interquartile values of the measured responses. Note that in some panels, the thick and thin traces are more or less indiscernible (GIF 106 KB)
Fig. S1
High resolution image file (EPS 2.35 MB)
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Schmidt, H., Eilers, J. Spine neck geometry determines spino-dendritic cross-talk in the presence of mobile endogenous calcium binding proteins. J Comput Neurosci 27, 229–243 (2009). https://doi.org/10.1007/s10827-009-0139-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-009-0139-5