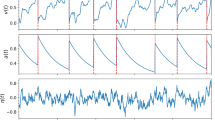
Abstract
We discuss how to characterize long-time dynamics of non-smooth dynamical systems, such as integrate-and-fire (I&F) like neuronal network, using Lyapunov exponents and present a stable numerical method for the accurate evaluation of the spectrum of Lyapunov exponents for this large class of dynamics. These dynamics contain (i) jump conditions as in the firing-reset dynamics and (ii) degeneracy such as in the refractory period in which voltage-like variables of the network collapse to a single constant value. Using the networks of linear I&F neurons, exponential I&F neurons, and I&F neurons with adaptive threshold, we illustrate our method and discuss the rich dynamics of these networks.










Similar content being viewed by others
References
Alstrom, P., Christiansen, B., & Levinsen, M. T. (1988). Nonchaotic transition from quasiperiodicity to complete phase locking. Physical Review Letters, 61, 1679–1682.
Azouz, R., & Gray, C. M. (1999). Cellular mechanisms contributing to response variability of cortical neurons in vivo. Journal of Neuroscience, 19, 2209–2223.
Benettin, G., Galgani, L., Giorgilli, A., & Strelcyn, J. M. (1980a). Lyapunov Characteristic Exponents for smooth dynamical systems and for hamiltonian systems; A method for computing all of them, Part I: Theory. Meccanica, 15, 9–20.
Benettin, G., Galgani, L., Giorgilli, A., & Strelcyn, J. M. (1980b). Lyapunov Characteristic Exponents for smooth dynamical systems and for hamiltonian systems; A method for computing all of them, Part II: Numerical application. Meccanica, 15, 21–30.
Brette, R. (2004). Dynamics of one-dimensional spiking neuron models. Journal of Mathematical Biology, 48, 38–56.
Brette, R., & Gerstner, W. (2005). Adaptive exponential integrate-and-fire model as an effective description of neuronal activity. Journal of Mathematical Biology, 94, 3637–3642.
Burkitt, A. N. (2006a). A review of the integrate-and-fire neuron model: I. Homogeneous synaptic input. Biological Cybernetics, 95, 1–19.
Burkitt, A. N. (2006b). A review of the integrate-and-fire neuron model: II. Inhomogeneous synaptic input and network properties. Biological Cybernetics, 95, 97–112.
Cai, D., Rangan, A. V., & McLaughlin, D. W. (2005). Architectural and synaptic mechanisms underlying coherent spontaneous activity in V1. Proceedings of National Academy of Sciences of the United States of America, 102, 5868–5873.
Carandini, M., Mechler, F., Leonard, C. S., & Movshon, J. A. (1996). Spike train encoding by regular-spiking cells of the visual cortex. Journal of Neurophysiology, 76, 3425–3441.
Chacron, M. J., Lindner, B., & Longtin, A. (2007). Threshold fatigue and information transmission. Journal of Computational Neuroscience, 23, 301–311.
Chacron, M. J., Longtin, A., & Maler, L. (2001a). Negative interspike interval correlations increase the neuronal capacity for encoding time dependent stimuli. Journal of Neuroscience, 21, 5328–5343.
Chacron, M. J., Longtin, A., & Maler, L. (2001b). Simple models of bursting and non-bursting P-type electroreceptors. Neurocomputing, 38, 129–139.
Chacron, M. J., Longtin, A., & Pakdaman, K. (2004). Chaotic firing in the sinusoidally forced leaky integrate-and-fire model with threshold fatigue. Physica D, 192, 138–160.
Chacron, M. J., Longtin, A., St-Hilaire, M., & Maler, L. (2000). Suprathreshold stochastic firing dynamics with memory in P-type electroreceptors. Physical Review Letters, 85, 1576–1579.
Chacron, M. J., Pakdaman, K., & Longtin, A. (2003). Interspike interval correlations, memory, adaptation, and refractoriness in a leaky integrate-and-fire model with threshold fatigue. Neural Computation, 15, 253–276.
Dayan, P., & Abbott, L. F. (2001). Theoretical Neuroscience. Massachusetts: MIT.
de Souza, S. L. T., & Caldas, I. L. (2001). Basins of attraction and transient chaos in a gear-rattling model. Journal of Vibration and Control, 7, 849–862.
de Souza, S. L. T., & Caldas, I. L. (2004). Calculation of Lyapunov exponents in systems with impacts. Chaos, Solitons & Fractals, 19, 569–579.
Eckmann, J. P., & Ruelle, D. (1985). Ergodic theory of chaos and strange attractors. Reviews of Modern Physics, 57, 617–656.
Farmer, J. D., Ott, E., & Yorke, J. A. (1983). The dimension of chaotic attractors. Physica D, 7, 153–180.
Fourcaud-Trocme, N., Hansel, D., van Vreeswijk, C., & Brunel, N. (2003). How spike generation mechanisms determine the neuronal response to fluctuating inputs. Journal of Neuroscience, 23, 11628–11640.
Frederickson, P., Kaplan, J. L., Yorke, E. D., & Yorke, J. A. (1983). The Lyapunov dimension of strange attractors. Journal of Differential Equations, 49, 185–207.
Fujisaka, H., & Yamada, T. (1983). Stability theory of synchronized motion in coupled-oscillator systems. Progress of Theoretical Physics, 69, 32–47.
Geisler, C., Brunel, N., & Wang, X. J. (2005). Contributions of intrinsic membrane dynamics to fast network oscillations with irregular neuronal discharges. Journal of Neurophysiology, 94, 4344–4361.
Gerstner, W., & Kistler, W. M. (2002). Spiking neuron models. Cambridge: Cambridge University Press.
Guckenheimer, J., & Holmes, P. (1983). Nonlinear oscillations, dynamical systems, and bifurcations of vector fields. New York: Springer.
Han, R. P. S., Luo, A. C. J., & Deng, W. (1995). Chaotic motion of a horizontal impact pair. Journal of Sound & Vibration, 181, 231–250.
Harsch, A., & Robinson, H. P. (2000). Postsynaptic variability of firing in rat cortical neurons: The roles of input synchronization and synaptic NMDA receptor conductance. Journal of Neuroscience, 20, 6181–6192.
Hegger, R., Kantz, H., & Schreiber, T. (1999). Practical implementation of nonlinear time series methods: The TISEAN package. Chaos, 9, 413–435.
Hinrichs, N., Oestreich, M., & Popp, K. (1997). Dynamics of oscillators with impact and friction. Chaos, Solitons & Fractals, 8, 535–558.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology, 117, 500–544.
Horn, D., & Opher, I. (2000). Complex dynamics of neuronal thresholds. Neurocomputing, 32, 161–166.
Horn, D., & Usher, M. (1989). Neural networks with dynamical thresholds. Physical Review A, 40, 1036–1044.
Kantz, H. (1994). A robust method to estimate the maximal Lyapunov exponent of a time series. Physics letters A, 185, 77–87.
Kaplan, J. L., & Yorke, J. A. (1979). Funtional differential equations and approximations of fixed points. Berlin: Springer.
Lichtenberg, A. L., & Lieberman, M. A. (1992). Regular and chaotic dynamics. New York: Springer.
Lindner, B., & Longtin, A. (2005). Effect of an exponentially decaying threshold on the firing statistics of a stochastic integrate-and-fire neuron. Journal of Theoretical Biology, 232, 505–521.
Liu, Y. H., & Wang, X. J. (2001). Spike-frequency adaptation of a generalized integrate-and fire model neuron. Journal of Computational Neuroscience, 10, 25–45.
Mainen, Z. F., & Sejnowski, T. J. (1995). Reliability of spike timing in neocortical neurons. Science, 268, 1503–1506.
McLaughlin, D. W., Shapley, R., Shelley, M., & Wielaard, D. J. (2000). A neuronal network model of macaque primary visual cortex (V1): Orientation selectivity and dynamics in the input layer 4C alpha. Proceedings of National Academy of Sciences of the United States of America, 97, 8087–8092.
Mueller, P. C. (1995). Calculation of Lyapunov exponents for dynamics systems with discontinuities. Chaos, Solitons & Fractals, 5, 1671–1681.
Nowak, L. G., Azouz, R., Sanchez-vives, M. V., Gray, C. M., & McCormick, D. A. (2003). Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. Journal of Neurophysiology, 89, 1541–1566.
Oseledec, V. I. (1968). A multiplicative ergodic theorem: Lyapunov characteristic numbers for dynamical systems. Transactions of the Moscow Mathematical Society, 19, 197–221.
Ott, E. (1993). Chaos in dynamical systems. New York: Cambridge University Press.
Parker, T. S., & Chua, L. O. (1989). Practical numerical algorithms for chaotic systems. New York: Springer.
Pecora, L. M., & Carroll, T. L. (1990). Synchronization in chaotic systems. Physical Review Letters, 64, 821–824.
Rabinovich, M. I., Varona, P., Selverston, A. I., & Abarbanel, H. D. I. (2006). Dynamical principles in neuroscience. Reviews of Modern Physics, 78, 1213–1265.
Ramasubramanian, K., & Seiram, M. S. (2000). A comparative study of computation of Lyapunov spectra with different algorithms. Physica D, 139, 72–86.
Rangan, A. V., & Cai, D. (2007). Fast numerical methods for simulating large-scale integrate-and-fire neuronal networks. Journal of Computational Neuroscience, 22, 81–100.
Rangan, A. V., Cai, D., & McLaughlin, D. W. (2005). Modeling the spatiotemporal cortical activity associated with the line-motion illusion in primary visual cortex. Proceedings of National Academy of Sciences of the United States of America, 102, 18793–18800.
Rauch, A., Camera, G. L., Luscher, H., Senn, W., & Fusi, S. (2003). Neocortical pyramidal cells respond as integrate-and-fire neurons to in vivo-like input currents. Journal of Neurophysiology, 90, 15980–1612.
Shadlen, M. N., & Newsome, W. T. (1998). The variable discharge of cortical neurons: Implications for connectivity, computation and information coding. Journal of Neuroscience, 18, 3870–3896.
Softky, W. R., & Koch, C. (1993). The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. Journal of Neuroscience, 13, 334–350.
Somers, D. C., Nelson, S. B., & Sur, M. (1995). An emergent model of orientation selectivity in cat visual cortical simple cells. Journal of Neuroscience, 15, 5448–5465.
Stefanski, A. (2000). Estimation of the largest Lyapunov exponent in systems with impacts. Chaos, Solitons & Fractals, 11, 2443–2451.
Stefanski, A., & Kapitaniak, T. (2000). Using chaos synchronization to estimate the largest Lyapunov exponent of nonsmooth systems. Discrete Dynamics in Nature and Society, 4, 207–215.
Stefanski, A., & Kapitaniak, T. (2003). Estimation of the dominant Lyapunov exponent of non-smooth systems on the basis of maps synchronization. Chaos, Solitons & Fractals, 15, 233–244.
Tao, L., Shelley, M., McLaughlin, D. W., & Shapley, R. (2004). An egalitarian network model for the emergence of simple and complex cells in visual cortex. Proceedings of National Academy of Sciences of the United States of America, 101, 366–371.
Tolhurst, D. J., Movshon, J. A., & Dean, A. F. (1983). The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Research, 23, 775–785.
Troyer, T. W., Krukowski, A. E., Priebe, N. J., & Miller, K. D. (1998). Contrast-invariant orientation tuning in cat visual cortex: Thalamocortical input tuning and correlation-based intracortical connectivity. Journal of Neuroscience, 18, 5908–5927.
Wang, X. J., & Buzsaki, G. (1996). Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. Journal of Neuroscience, 16, 6402–6413.
Wark, B., Lundstrom, B. N., & Fairhall, A. (2007). Sensory adaptation. Current opinion in Neurobiology, 17, 423–429.
Wolf, A., Swift, J. B., Swinney, H. L., & Vastano, J. A. (1985). Determining lyapunov exponents from a time series. Physica D, 16, 285–317.
Zhang, L., & McBain, C. J. (1995). Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. Journal of Physiology, 488, 661–672.
Acknowledgements
The work was supported by NSF grants and a grant from the Swartz foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: David Terman
Appendix: Proof of no degeneracy in the augmented dynamics
Appendix: Proof of no degeneracy in the augmented dynamics
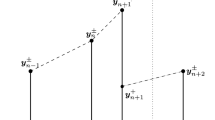
Here, we demonstrate that the introduction of the dynamics of the refractory residence time indeed extends the original standard algorithm of computing LEs and does not encounter the degeneracy problem as discussed before. In other words, we can show that evolving from an initial ball with radius ε centered at the reference trajectory to any time T, one can always obtain a linear transformed ellipsoid without degeneracy.
Suppose at time T (n) = nΔ T, the system is in the network subthreshold period and the perturbation vector \(\delta{\boldsymbol{x}}^{(n)}\) is given as \((\delta{\boldsymbol{x}}^{(n)}_1, \delta{\boldsymbol{x}}^{(n)}_2,\cdots,\delta{\boldsymbol{x}}^{(n)}_N)\), where \(\delta{\boldsymbol{x}}^{(n)}_i\!=\!(\delta{V}_i^{(n)},\delta{G}_i^{(n)})\). Then, at the next time T (n + 1) = (n + 1)ΔT, the system is in the network refractory period with the perturbation vector \(\delta{\boldsymbol{x}}^{(n+1)}\!=\) \((\delta{\boldsymbol{x}}^{(n+1)}_1, \delta{\boldsymbol{x}}^{(n+1)}_2,\cdots,\delta{\boldsymbol{x}}^{(n+1)}_N)\), where \(\delta{\boldsymbol{x}}^{(n+1)}_i \!=\! (\delta{\tau}_i^{(n+1)},\) \( \delta{G}_i^{(n+1)})\) if \(T^{(n+1)}\in \bigcup\limits_k(T_{i,k},T_{i,k}+\tau_{\rm{ref}})\) and \(\delta{\boldsymbol{x}}^{(n+1)}_i = (\delta{V}_i^{(n+1)},\delta{G}_i^{(n+1)})\) if \(T^{(n+1)}\in\bigcup\limits_k(T_{i,k}+\tau_{\rm{ref}},\,T_{i,k+1})\). We show that there is no degeneracy problem when using GSR at time T (n + 1) to obtain the local LEs of the system. In other words, the evolution matrix \(\mathbb{A}^{(n,n+1)}\) over the interval [T (n),T (n + 1)] linking the perturbation vector from \(\delta{\boldsymbol{x}}^{(n)}\) to \(\delta{\boldsymbol{x}}^{(n+1)}\) is nondegenerate (nonsingular). We first divide the interval [T (n),T (n + 1)] into the combination of several subintervals as \([T^{(n)},T^{(n+1)}]\!=\!\bigcup\limits_{i=0}^{M-1}\bar{E}_{i}\), where \(\bar{E}_i\!=\![T^{(n_i)},\) \(T^{(n_{i+1})}]\) with n 0 = n, n M = n + 1, and \(T^{(n)}<T^{(n_i)}<T^{(n+1)}\,(i=1,2,\cdots,M-1)\) are chosen from either the set of neurons’ spike time \(\bigcup\limits_j\bigcup\limits_k\{T_{j,k}\}\) or the set of neurons’ awakening time \(\bigcup\limits_j\bigcup\limits_k\{T_{j,k}+\tau_{\rm{ref}}\}\). Notice that the dynamics of the perturbation vector over each subinterval \(E_i=(T^{(n_i)},T^{(n_{i+1})})\) is smooth, we will show that both the evolution matrix \(\mathbb{B}^{(n_i,n_{i+1})}\) over each subinterval linking the perturbation vector from \(\delta{\boldsymbol{x}}^{(n_i^{+})}\) to \(\delta{\boldsymbol{x}}^{(n_{i+1}^{-})}\) and the transition matrix \(\mathbb{R}^{(n_i}\) immediately before and after each \(T^{(n_i)}\) linking the perturbation vector from \(\delta{\boldsymbol{x}}^{(n_i^{-})}\) to \(\delta{\boldsymbol{x}}^{(n_i^{+})}\) are nondegenerate, where \(\delta{\boldsymbol{x}}^{(n_i^{+})}\) and \(\delta{\boldsymbol{x}}^{(n_{i+1}^{-})}\) represent one-sided limits of the perturbation vector \(\delta{\boldsymbol{x}}\) obtained as \(\lim\limits_{{T-T^{(n_i)}\rightarrow{0^{+}}}}\delta{\boldsymbol{x}}(T)\) and \(\lim\limits_{{T-T^{(n_{i+1})}\rightarrow{0^{-}}}}\delta{\boldsymbol{x}}(T)\). For any n i (i = 1,2, ⋯ ,M − 1), we first consider the transition matrix \(\mathbb{R}^{(n_i}\) immediately before and after \(T^{(n_i)}\). (i) If \(T^{(n_i)}=T_{j,k}\) (the kth spike time of the jth neuron), the transition matrix \(\mathbb{R}^{(n_i}\) can be obtained as
where \(\mathbb{R}^{(n_i}\) represents the element of the matrix with the position at the pth row and the qth column. The first line on the RHS of Eq. (20) describes the linear transition coefficient of the lth neuron’s voltage component in the perturbation vector linking \(\delta{V}_l^{(n_i^{-})}\) and \(\delta{V}_l^{(n_i^{+})}\) with the label l ≠ j. The second line on the RHS of Eq. (20) describes the linear transition coefficient of the jth neuron’s voltage component in the perturbation vector linking \(\delta{V}_j^{(n_i^{-})}\) and \(\delta{\tau}_j^{(n_i^{+})}\) as in Eq. (13). The third line combined with the first line on the RHS of Eq. (20) describes the linear transition coefficients of the lth neuron’s conductance component in the perturbation vector linking \(\delta{G}_l^{(n_i^{-})}\) and \(\delta{G}_l^{(n_i^{+})}\) with the label l ≠ j as in Eq. (10) (we only consider the effects of the jth neuron’s spike to the change of other neurons’ conductance, not to itself). (ii) If \(T^{(n_i)}=T_{j,k}+\tau_{\rm{ref}}\) (the kth awakening time of the jth neuron), the transition matrix \(\mathbb{R}^{(n_i}\) can be obtained as
where the first line on the RHS of Eq. (21) describes the linear transition coefficient of the lth neuron’s voltage component in the perturbation vector linking \(\delta{V}_l^{(n_i^{-})}\) and \(\delta{V}_l^{(n_i^{+})}\) with the label l ≠ j, and also the linear transition coefficient of the lth neuron’s conductance component in the perturbation vector linking \(\delta{G}_l^{(n_i^{-})}\) and \(\delta{G}_l^{(n_i^{+})}\) with the label l = 1,2, ⋯ ,N. The second line on the RHS of Eq. (21) describes the linear transition coefficient of the jth neuron’s voltage component in the perturbation vector linking \(\delta{\tau}_j^{(n_i^{-})}\) and \(\delta{V}_j^{(n_i^{+})}\) as in Eq. (14). We can see that the transition matrix is nondegenerate in both cases (i) and (ii). Next, we consider the evolution matrix \(\mathbb{B}^{(n_i,n_{i+1})}\) over each subinterval E i . For any n i (i = 0,1, ⋯ ,M − 1), we define two sets of neuron index for a given E i as follows: the first set I is given as \(I=\{j|1\leq{j}\leq{N},\,E_i\subseteq{\Gamma_j}\}\), where Γ j is the set of the jth neuron’s refractory period as \(\Gamma_j=\bigcup\limits_k(T_{j,k},T_{j,k}+\tau_{\rm{ref}})\). The second set J is given as \(J=\{j|1\leq{j}\leq{N},\,E_i\subseteq{\Lambda_j}\}\) , where Λ j is the set of the jth neuron’s subthreshold period as \(\Lambda_j=\bigcup\limits_k(T_{j,k}+\tau_{\rm{ref}},T_{j,k+1})\). The evolution matrix \(\mathbb{B}^{(n_i,n_{i+1})}\) can be obtained as \(\mathbb{B}^{(n_i,n_{i+1})}(p,q) =\)
where
The first line and the second line on the RHS of Eq. (22) describe the linear transition coefficients of the lth neuron’s voltage component in the perturbation vector linking \(\delta{V}_l^{(n_i^{-})}\), \(\delta{G}_l^{(n_i^{-})}\) and \(\delta{V}_l^{(n_i^{+})}\) as obtained by linearization of Eq. (5) with the label l ∈ J (i.e., the neuron under its subthreshold dynamics). The third line on the RHS of Eq. (22) describes the linear transition coefficient of the lth neuron’s conductance component in the perturbation vector linking \(\delta{G}_l^{(n_i^{-})}\) and \(\delta{G}_l^{(n_i^{+})}\) with the label l = 1,2, ⋯ ,N. The fourth line on the RHS of Eq. (22) describes the linear transition coefficient of the lth neuron’s voltage component in the perturbation vector linking \(\delta{\tau}_l^{(n_i^{-})}\) and \(\delta{\tau}_l^{(n_i^{+})}\) with the label l ∈ I (i.e., the neuron under its refractory dynamics). We can see that the evolution matrix \(\mathbb{B}^{(n_i,n_{i+1})}\) is also nondegenerate. The evolution matrix \(\mathbb{A}^{(n)}\) over the whole interval [T (n),T (n + 1)] should be the product of both the evolution matrix over each subinterval \((T^{(n_i)},T^{(n_{i+1})})\) and the transition matrix immediately before and after each \(T^{(n_i)}\) as \(\mathbb{A}^{(n)}=\mathbb{B}^{(n_0,n_1)}\mathbb{R}^{(n_1)}\times \mathbb{B}^{(n_1,n_2)}\mathbb{R}^{(n_2)}\cdots\mathbb{R}^{(n_{M-1})} \mathbb{B}^{(n_{M-1},n_M)}\). Therefore, the evolution matrix \(\mathbb{A}^{(n)}\) is nondegenerate and we will not encounter the degeneracy problem when computing the local LEs using GSR. This is because we can always generate a complete set of corresponding orthogonal vectors at time T (n + 1) even though T (n + 1) is in a network refractory period. As a conclusion, both the theoretical definition and the numerical algorithm of LEs for smooth dynamical systems can be extended to the non-smooth dynamical systems of I&F networks with refractory-induced degeneracy.
Rights and permissions
About this article
Cite this article
Zhou, D., Sun, Y., Rangan, A.V. et al. Spectrum of Lyapunov exponents of non-smooth dynamical systems of integrate-and-fire type. J Comput Neurosci 28, 229–245 (2010). https://doi.org/10.1007/s10827-009-0201-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-009-0201-3