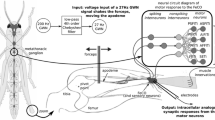
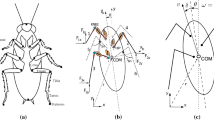
Abstract
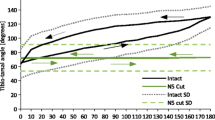
Nonlinear type system identification models coupled with white noise stimulation provide an experimentally convenient and quick way to investigate the often complex and nonlinear interactions between the mechanical and neural elements of reflex limb control systems. Previous steady state analysis has allowed the neurons in such systems to be categorised by their sensitivity to position, velocity or acceleration (dynamics) and has improved our understanding of network function. These neurons, however, are known to adapt their output amplitude or spike firing rate during repetitive stimulation and this transient response may be more important than the steady state response for reflex control. In the current study previously used system identification methods are developed and applied to investigate both steady state and transient dynamic and nonlinear changes in the neural circuit responsible for controlling reflex movements of the locust hind limbs. Through the use of a parsimonious model structure and Monte Carlo simulations we conclude that key system dynamics remain relatively unchanged during repetitive stimulation while output amplitude adaptation is occurring. Whilst some evidence of a significant change was found in parts of the systems nonlinear response, the effect was small and probably of little physiological relevance. Analysis using biologically more realistic stimulation reinforces this conclusion.











Similar content being viewed by others
References
Aertsen, A. M. H. J., & Johannesma, P. I. M. (1981). The spectro-temporal receptive field. Biological Cybernetics, 42, 133–143.
Bassler, U. (1993). The femur-tibia control system of stick insects—a model system for the study of the neural basis of joint control. Brain Research Reviews, 18(2), 207–226.
Benda, J., Maler, L., & Longtin, A. (2010). Linear versus nonlinear signal transmission in neuron models with adaptation currents or dynamic thresholds. Journal of Neurophysiology, 104(5), 2806–2820.
Blackburn, L. M., Ott, S. R., Matheson, T., Burrows, M., & Rogers, S. M. (2010). Motor neurone responses during a postural reflex in solitarious and gregarious desert locusts. Journal of Insect Physiology, 56(8), 902–910.
Brenner, N., Bialek, W., & de Ruyter van Steveninck, S. (2000). Adaptive rescaling maximizes information transmission. Neuron, 26(3), 695–702.
Burrows, M. (1996). The neurobiology of an insect brain (1st ed.). Oxford University Press.
Burrows, M., & Morris, G. (2001). The kinematics and neural control of high-speed kicking movements in the locust. Journal of Experimental Biology, 204(20), 3471–3481.
Burrows, M., & Hoyle, G. (1973). Neural mechanism underlying behaviour in the locust Schistocerca gregaria III. Topography of limb motorneurons in the metathoracic ganglion. Journal of Neurobiology, 4(2), 167–186.
Burrows, M., & Matheson, T. (1994). A presynaptic gain control mechanism among sensory neurons of a locust leg proprioceptor. Journal of Neuroscience, 12(1), 272–282.
Dewhirst, O. P., Angarita-James, N., Simpson, D., Allen, R., & Newland, P. (2011). The dynamics of locust non-spiking local interneurons, responses to imposed limb movements. In Proceedings of biosignals 2011 international conference on bio-inspired systems and signal processing (pp. 270–275). Rome, Italy.
Doucet, A., De Freitas, N., & Gordon, N. (2001). Sequential Monte Carlo methods in practice (1st ed.). Springer.
Eggermont, J. J. (1993). Wiener and Volterra analyses applied to the auditory system. Hearing Research, 66(2), 177–201.
Field, L. H., & Burrows, M. (1982). Reflex effects of the femoral chordotonal organ upon leg motor neurones of the locust. Journal of Experimental Biology, 101(1), 265–285.
Field, L. H., & Matheson, T. (1998). Chordotonal organs of insects. Advances in Insect Physiology, 27(27), 1–228.
French, A. S. (1986). The role of calcium in the rapid adaptation of an insect mechanoreceptor. Journal of Neuroscience, 6(8), 2322–2326.
French, A. S., & Korenberg, M. J. (1989). A nonlinear cascade model for action potential encoding in an insect sensory neuron. Biophysical Journal, 55(4), 655–661.
French, A. S., Sekizawa, S., Höger, U., & Torkkeli, P. H. (2001). Predicting the responses of mechanoreceptor neurons to physiological inputs by nonlinear system identification. Annals of Biomedical Engineering, 29(3), 187–194.
Gamble, E. R., & DiCaprio, R. A. (2003). Nonspiking and spiking proprioceptors in the crab: White noise analysis of spiking CB-chordotonal organ afferents. Journal of Neurophysiology, 89(4), 1815–1825.
Hildebrandt, K. J., Benda, J., & Hennig, R. M. (2009). The origin of adaptation in the auditory pathway of locusts is specific to cell type and function. Journal of Neuroscience, 29(8), 2626–2636.
Hoyle, G., & Burrows, M. (1973). Neural mechanism underlying behaviour in the locust Schistocerca gregaria I. Physiology of identified motorneurons in the metathoracic ganglion. Journal of Neurobiology, 4(1), 3–41.
Hoyle, M. (1953). Potassium ions and insect nerve muscle. Journal of Experimental Biology, 30(1), 121–135.
Hunter, I. W., & Korenberg, M. J. (1986). The identification of nonlinear biological systems: Wiener and Hammerstein cascade models. Biological Cybernetics, 55(2), 135–144.
Ingham, N. J., & McAlpine, D. (2005). GABAergic inhibition controls neural gain in inferior colliculus neurons sensitive to interaural time differences. Journal of Neuroscience, 25(26), 6187–6198.
Jaaskelainen, I. P., Ahveninen, J., Bonmassar, G., Dale, A. M., Ilmoniemi, R. J., Levänen, S., et al. (2004). Human posterior auditory cortex gates novel sounds to consciousness. Proceedings of the National Academy of Sciences of the United States of America, 101(17), 6809–6814.
Kay, S. M. (1988). Modern spectral estimation: Theory and application. Prentice-Hall.
Kittmann, R. (1997). Neural mechanisms of adaptive gain control in a joint control loop: Muscle force and motorneuronal activity. Journal of Experimental Biology, 200, 1383–1402.
Kondoh, Y., Okuma, J., & Newland, P. L. (1995). Dynamics of neurons controlling movements of a locust hind leg: Wiener kernel analysis of the responses of the proprioceptive afferents. Journal of Neurophysiology, 73(5), 1829–1842.
Korenberg, M. J., & Hunter, I. W. (1986). The identification of nonlinear biological systems: LNL cascade models. Biological Cybernetics, 50(2), 125–134.
Kruskal, W. H., & Wallis, W. A. (1952). Use of Ranks in one-criterion variance analysis. Journal of the American Statistical Association, 47(260), 583–621.
Laughlin, S. B. (1989). The role of sensory adaptation in the retina. Journal of Experimental Biology, 146(1), 39–62.
Lee, Y. W., & Schetzen, M. (1965). Measurement of the Wiener kernels of a nonlinear system by cross-correlation. International Journal of Control, 2, 237–254.
Mainardi, L. T. (2009). On the quantification of heart rate variability spectral parameters using time-frequency and time-varying methods. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 367(1887), 255–275.
Marmarelis, V. Z. (1993). Identification of nonlinear biological systems using Laguerre expansions of kernels. Annals of Biomedical Engineering, 21, 573–589.
Marmarelis, V. Z. (2004). Nonlinear dynamic modeling of physiological systems (1st ed.). Wiley-IEEE Press.
Marquardt, D. W. (1963). An algorithm for Least-squares estimation of nonlinear parameters. SIAM Journal on Applied Mathematics, 11(2), 431–441.
McDonald, J. H. (2009). Handbook of biological statistics (2nd ed.). Sparky House Publishing.
Nelles, O. (2001). Nonlinear system identification (1st ed.). Springer Verlag.
Newland, P. L. (1991). Physiological properties of afferents from tactile hairs on the hind legs of the locust. Journal of Experimental Biology, 155, 487–503.
Newland, P. L., & Kondoh, Y. (1997a). Dynamics of neurons controlling movements of a locust hind leg II: Flexor tibiae motor neurons. Journal of Neurophysiology, 77(4), 1731–1746.
Newland, P. L., & Kondoh, Y. (1997b). Dynamics of neurons controlling movements of a locust hind leg III: Extensor tibiae motor neurons. Journal of Neurophysiology, 77(5), 3297–3310.
Prescott, S. A., & Sejnowski, T. J. (2008). Spike-rate coding and spike-time coding are affected oppositely by different adaptation mechanisms. Journal of Neuroscience, 28(50), 13649–13661.
Schwarzenbach, J., & Allen, R. (1980). Modelling of physiological receptors using on line data acquisition. In R. Isermann (Ed.), Proceedings of 5th IFAC symposium on ’identification and system parameter estimation’ Darmstadt, Fed. Rep. Germany, 24–28 Sept 1979 (Vol. 2, pp. 817–826). Oxford: Pergamon Press.
Theunissen, F. E., David, S. V., Singh, N. C., Hsu, A., Vinje, W. E., & Gallant, J. L. (2001). Estimating spatio-temporal receptive fields of auditory and visual neurons from their responses to natural stimuli. Network, 12(3), 289–316.
Touryan, J., Felson, G., & Dan, Y. (2005). Spatial structure of complex cell receptive fields measured with natural images. Neuron, 45(5), 781–791.
Vidal-Gadea, A. G., Jing, X., Simpson, D., Dewhirst, O. P., Kondoh, Y., Allen, R., et al. (2010). Coding characteristics of spiking local interneurons during imposed limb movements in the locust. Journal of Neurophysiology, 103, 603–615.
Westwick, D. T., & Kearney, R. E. (2006). Identification of nonlinear physiological systems (1st ed.). IEEE Press.
Wu, M. C. K., David, S. V., & Gallant, J. L. (2006). Complete functional characterization of sensory neurons by system identification. Annual Review of Neuroscience, 29(1), 477–505.
Acknowledgements
This work was supported with awards from the Biotechnology and Biological Sciences Research Council (UK), the EPSRC and the Gerald Kerkut Charitable Trust (UK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Aurel A. Lazar
Rights and permissions
About this article
Cite this article
Dewhirst, O.P., Angarita-Jaimes, N., Simpson, D.M. et al. A system identification analysis of neural adaptation dynamics and nonlinear responses in the local reflex control of locust hind limbs. J Comput Neurosci 34, 39–58 (2013). https://doi.org/10.1007/s10827-012-0405-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-012-0405-9