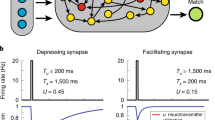
Abstract
Models for temporary information storage in neuronal populations are dominated by mechanisms directly dependent on synaptic plasticity. There are nevertheless other mechanisms available that are well suited for creating short-term memories. Here we present a model for working memory which relies on the modulation of the intrinsic excitability properties of neurons, instead of synaptic plasticity, to retain novel information for periods of seconds to minutes. We show that it is possible to effectively use this mechanism to store the serial order in a sequence of patterns of activity. For this we introduce a functional class of neurons, named gate interneurons, which can store information in their membrane dynamics and can literally act as gates routing the flow of activations in the principal neurons population. The presented model exhibits properties which are in close agreement with experimental results in working memory. Namely, the recall process plays an important role in stabilizing and prolonging the memory trace. This means that the stored information is correctly maintained as long as it is being used. Moreover, the working memory model is adequate for storing completely new information, in time windows compatible with the notion of “one-shot” learning (hundreds of milliseconds).








Similar content being viewed by others
References
Abbott, L. F., & Nelson, S. B. (2000). Synaptic plasticity: taming the beast. Nature Neuroscience, 3(Suppl), 1178–1183.
Aguiar, P., Sousa, M., & Lima, D. (2010). NMDA channels together with L-type calcium currents and calcium-activated nonspecific cationic currents are sufficient to generate windup in WDR neurons. Journal of Neurophysiology, 104, 1155–1166.
Alonso, A., de Curtis, M., & Llinas, R. (1990). Postsynaptic Hebbian and non-Hebbian long-term potentiation of synaptic efficacy in the entorhinal cortex in slices and in the isolated adult guinea pig brain. Proceedings of the National Academy of Sciences of the United States of America, 87, 9280–9284.
Baddeley, A. (2003). Working memory: looking back and looking forward. Nature Reviews Neuroscience, 4, 829–839.
Bal, T., & McCormick, D. A. (1993). Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. The Journal of Physiology, 468, 669–691.
Bliss, T. V., & Lomo, T. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of Physiology, 232, 331–356.
Botvinick, M. M., & Plaut, D. C. (2006). Short-term memory for serial order: a recurrent neural network model. Psychology Review, 113, 201–233.
Burnashev, N., Zhou, Z., Neher, E., & Sakmann, B. (1995). Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. The Journal of Physiology, 485(Pt 2), 403–418.
Buxhoeveden, D. P., & Casanova, M. F. (2002). The minicolumn hypothesis in neuroscience. Brain, 125, 935–951.
Crasto, C. J., Marenco, L. N., Liu, N., Morse, T. M., Cheung, K. H., Lai, P. C., et al. (2007). SenseLab: new developments in disseminating neuroscience information. Briefings in Bioinformatics, 8, 150–162.
Dehaene, S., Changeux, J. P., & Nadal, J. P. (1987). Neural networks that learn temporal sequences by selection. Proceedings of the National Academy of Sciences of the United States of America, 84, 2727–2731.
Destexhe, A., Babloyantz, A., & Sejnowski, T. J. (1993). Ionic mechanisms for intrinsic slow oscillations in thalamic relay neurons. Biophysical Journal, 65, 1538–1552.
Destexhe, A., Contreras, D., Sejnowski, T. J., & Steriade, M. (1994). A model of spindle rhythmicity in the isolated thalamic reticular nucleus. Journal of Neurophysiology, 72, 803–818.
Durstewitz, D., Seamans, J. K., & Sejnowski, T. J. (2000). Neurocomputational models of working memory. Nature Neuroscience, 3(Suppl), 1184–1191.
Fall, C. P., & Rinzel, J. (2006). An intracellular Ca2+ subsystem as a biologically plausible source of intrinsic conditional bistability in a network model of working memory. Journal of Computational Neuroscience, 20, 97–107.
Feldmeyer, D., Egger, V., Lubke, J., & Sakmann, B. (1999). Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel’ of developing rat somatosensory cortex. The Journal of Physiology, 521(Pt 1), 169–190.
Fransen, E., Alonso, A. A., & Hasselmo, M. E. (2002). Simulations of the role of the muscarinic-activated calcium-sensitive nonspecific cation current INCM in entorhinal neuronal activity during delayed matching tasks. Journal of Neuroscience, 22, 1081–1097.
Fransen, E., Tahvildari, B., Egorov, A. V., Hasselmo, M. E., & Alonso, A. A. (2006). Mechanism of graded persistent cellular activity of entorhinal cortex layer v neurons. Neuron, 49, 735–746.
Gibson, W. G., & Robinson, J. (1992). Statistical analysis of the dynamics of a sparse associative memory. Neural Networks, 5, 645–661.
Grashow, R., Brookings, T., & Marder, E. (2010). Compensation for variable intrinsic neuronal excitability by circuit-synaptic interactions. Journal of Neuroscience, 30, 9145–9156.
Hahn, T. T., McFarland, J. M., Berberich, S., Sakmann, B., & Mehta, M. R. (2012). Spontaneous persistent activity in entorhinal cortex modulates cortico-hippocampal interaction in vivo. Nature Neuroscience, 15, 1531–1538.
Haj-Dahmane, S., & Andrade, R. (1999). Muscarinic receptors regulate two different calcium-dependent non-selective cation currents in rat prefrontal cortex. European Journal of Neuroscience, 11, 1973–1980.
Herz, A. V., Li, Z., & van Hemmen, J. L. (1991). Statistical mechanics of temporal association in neural networks with transmission delays. Physical Review Letters, 66, 1370–1373.
Hines, M. L., & Carnevale, N. T. (1997). The NEURON simulation environment. Neural Computation, 9, 1179–1209.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of Physiology, 117, 500–544.
Hyde, R. A., & Strowbridge, B. W. (2012). Mnemonic representations of transient stimuli and temporal sequences in the rodent hippocampus in vitro. Nature Neuroscience, 15, 1430–1438.
Jackson, M. B., & Scharfman, H. E. (1996). Positive feedback from hilar mossy cells to granule cells in the dentate gyrus revealed by voltage-sensitive dye and microelectrode recording. Journal of Neurophysiology, 76, 601–616.
Jahr, C. E., & Stevens, C. F. (1990). Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. Journal of Neuroscience, 10, 3178–3182.
Jensen, O., Idiart, M. A., & Lisman, J. E. (1996). Physiologically realistic formation of autoassociative memory in networks with theta/gamma oscillations: role of fast NMDA channels. Learning and Memory, 3, 243–256.
Kandel, E. R. (2001). The molecular biology of memory storage: a dialogue between genes and synapses. Science, 294, 1030–1038.
Klink, R., & Alonso, A. (1997). Morphological characteristics of layer II projection neurons in the rat medial entorhinal cortex. Hippocampus, 7, 571–583.
Koene, R. A., & Hasselmo, M. E. (2007). First-in-first-out item replacement in a model of short-term memory based on persistent spiking. Cerebral Cortex, 17, 1766–1781.
Koene, R. A., & Hasselmo, M. E. (2008). Consequences of parameter differences in a model of short-term persistent spiking buffers provided by pyramidal cells in entorhinal cortex. Brain Research, 1202, 54–67.
Lisman, J. E., & Idiart, M. A. (1995). Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science, 267, 1512–1515.
Marder, E., Abbott, L. F., Turrigiano, G. G., Liu, Z., & Golowasch, J. (1996). Memory from the dynamics of intrinsic membrane currents. Proceedings of the National Academy of Sciences of the United States of America, 93, 13481–13486.
McCormick, D. A., & Huguenard, J. R. (1992). A model of the electrophysiological properties of thalamocortical relay neurons. Journal of Neurophysiology, 68, 1384–1400.
Mozzachiodi, R., & Byrne, J. H. (2010). More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends in Neurosciences, 33, 17–26.
Nagy, G. A., Botond, G., Borhegyi, Z., Plummer, N. W., Freund, T. F., & Hajos, N. (2012). DAG-sensitive and Ca(2+) permeable TRPC6 channels are expressed in dentate granule cells and interneurons in the hippocampal formation. Hippocampus.
Otis, T. S., De Koninck, Y., & Mody, I. (1993). Characterization of synaptically elicited GABAB responses using patch-clamp recordings in rat hippocampal slices. The Journal of Physiology, 463, 391–407.
Prinz, A. A., Bucher, D., & Marder, E. (2004). Similar network activity from disparate circuit parameters. Nature Neuroscience, 7, 1345–1352.
Suh, J., Rivest, A. J., Nakashiba, T., Tominaga, T., & Tonegawa, S. (2011). Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science, 334, 1415–1420.
Thomson, A. M., West, D. C., Wang, Y., & Bannister, A. P. (2002). Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2–5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cerebral Cortex, 12, 936–953.
Traub, R. D., & Miles, R. (1991). Neuronal networks of the hippocampus. Cambridge: CUP.
White, O. L., Lee, D. D., & Sompolinsky, H. (2004). Short-term memory in orthogonal neural networks. Physical Review Letters, 92, 148102.
Acknowledgments
Research funded by the European Regional Development Fund through the programme COMPETE and by the Portuguese Government through the FCT - Fundação para a Ciência e a Tecnologia under the project PEst-C/MAT/UI0144/2011. Eduardo Conde-Sousa was supported by the grant SFRH/BD/65633/2009 from FCT, co-financed by European Social Fund under the program POPH of the National Strategic Reference Framework.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: A. Borst
Rights and permissions
About this article
Cite this article
Conde-Sousa, E., Aguiar, P. A working memory model for serial order that stores information in the intrinsic excitability properties of neurons. J Comput Neurosci 35, 187–199 (2013). https://doi.org/10.1007/s10827-013-0447-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-013-0447-7