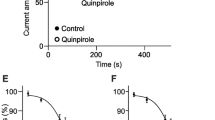
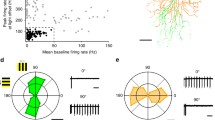
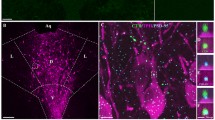
Abstract
Synchronized activities among retinal ganglion cells (RGCs) via gap junctions can be increased by exogenous dopamine (DA). During DA application, single neurons’ firing activities become more synchronized with its adjacent neighbors. One intriguing question is how the enhanced spatial synchronization alters the temporal firing structure of single neurons. In the present study, firing activities of bullfrog’s dimming detectors in response to binary pseudo-random checker-board flickering were recorded via a multi-channel recording system. DA was applied in the retina to modulate synchronized activities between RGCs, and the effect of DA on firing activities of single neurons was examined. It was found that, during application of DA, synchronized activities between single neuron and its neighboring neurons was enhanced. At the meantime, the temporal structures of single neuron spike train changed significantly, and the temporal correlation in single neuron’s response decreased. The pharmacological study results indicated that the activation of D1 receptor might have effects on gap junction permeability between RGCs. Our results suggested that the dopaminergic pathway participated in the modulation of spatial and temporal correlation of RGCs’ firing activities, and may exert critical effects on visual information processing in the retina.









Similar content being viewed by others
Abbreviations
- CCF:
-
Cross-correlation function
- DA:
-
Dopamine
- ISI:
-
Inter-spike-interval
- MEA:
-
Micro-electrode array
- RGC:
-
Retinal ganglion cell
- SCH:
-
SCH-23390
- SU:
-
Sulpiride
- TCI:
-
Temporal correlation index
References
Baccus, S. A., & Meister, M. (2002). Fast and slow contrast adaptation in retinal circuitry. Neuron, 36(5), 909–919.
Bloomfield, S. A., & Volgyi, B. (2009). The diverse functional roles and regulation of neuronal gap junctions in the retina. Nature Reviews Neuroscience, 10(7), 495–506.
Brivanlou, I. H., Warland, D. K., & Meister, M. (1998). Mechanisms of concerted firing among retinal ganglion cells. Neuron, 20(3), 527–539.
Buonomano, D. V., & Maass, W. (2009). State-dependent computations: Spatiotemporal processing in cortical networks. Nature Reviews Neuroscience, 10(2), 113–125.
Callier, S., Snapyan, M., Le Crom, S., Prou, D., Vincent, J. D., & Vernier, P. (2003). Evolution and cell biology of dopamine receptors in vertebrates. Biology of the Cell, 95(7), 489–502.
Chander, D., & Chichilnisky, E. J. (2001). Adaptation to temporal contrast in primate and salamander retina. Journal of Neuroscience, 21(24), 9904–9916.
Chen, A. H., Zhou, Y., Gong, H. Q., & Liang, P. J. (2004). Firing rates and dynamic correlated activities of ganglion cells both contribute to retinal information processing. Brain Research, 1017(1–2), 13–20. doi:10.1016/j.brainres.2004.04.081.
DeVries, S. H. (1999). Correlated firing in rabbit retinal ganglion cells. Journal of Neurophysiology, 81(2), 908–920.
Field, G. D., & Chichilnisky, E. J. (2007). Information processing in the primate retina: Circuitry and coding. Annual Review of Neuroscience, 30, 1–30. doi:10.1146/annurev.neuro.30.051606.094252.
Hampson, E. C., Vaney, D. I., & Weiler, R. (1992). Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. Journal of Neuroscience, 12(12), 4911–4922.
Hosoya, T., Baccus, S. A., & Meister, M. (2005). Dynamic predictive coding by the retina. Nature, 436(7047), 71–77.
Hu, E. H., Pan, F., Volgyi, B., & Bloomfield, S. A. (2010). Light increases the gap junctional coupling of retinal ganglion cells. The Journal of Physiology, 588(Pt 21), 4145–4163.
Jing, W., Liu, W. Z., Gong, X. W., Gong, H. Q., & Liang, P. J. (2010). Visual pattern recognition based on spatio-temporal patterns of retinal ganglion cells’ activities. Cogn Neurodyn, 4(3), 179–188. doi:10.1007/s11571-010-9119-8.
Koch, K., McLean, J., Berry, M., Sterling, P., Balasubramanian, V., & Freed, M. A. (2004). Efficiency of information transmission by retinal ganglion cells. Current Biology, 14(17), 1523–1530. doi:10.1016/j.cub.2004.08.060.
Krahe, R., & Gabbiani, F. (2004). Burst firing in sensory systems. Nature Reviews Neuroscience, 5(1), 13–23. doi:10.1038/nrn1296.
Lesica, N. A., & Stanley, G. B. (2004). Encoding of natural scene movies by tonic and burst spikes in the lateral geniculate nucleus. Journal of Neuroscience, 24(47), 10731–10740.
Lettvin, J. Y., Maturana, H. R., McCulloch, W. S., & Pitis, W. H. (1959). What the frog’s eye tells the Frog’s brain. Proceedings of the IRE, 47, 1940–1951.
Li, H., Liu, W. Z., & Liang, P. J. (2012). Adaptation-dependent synchronous activity contributes to receptive field size change of bullfrog retinal ganglion cell. PLoS One, 7(3), e34336. doi:10.1371/journal.pone.0034336.
Lisman, J. E. (1997). Bursts as a unit of neural information: Making unreliable synapses reliable. Trends in Neurosciences, 20(1), 38–43.
Liu, W. Z., Jing, W., Li, H., Gong, H. Q., & Liang, P. J. (2011a). Spatial and temporal correlations of spike trains in frog retinal ganglion cells. Journal of Computational Neuroscience, 30(3), 543–553. doi:10.1007/s10827-010-0277-9.
Liu, W. Z., Yan, R. J., Jing, W., Gong, H. Q., & Liang, P. J. (2011b). Spikes with short inter-spike intervals in frog retinal ganglion cells are more correlated with their adjacent neurons’ activites. Protein & Cell, 2, 764–771.
Liu, X., Zhou, Y., Gong, H. Q., & Liang, P. J. (2007). Contribution of the GABAergic pathway(s) to the correlated activities of chicken retinal ganglion cells. Brain Research, 1177, 37–46.
Meister, M., Lagnado, L., & Baylor, D. A. (1995). Concerted signaling by retinal ganglion cells. Science, 270(5239), 1207–1210.
Metzner, W., Koch, C., Wessel, R., & Gabbiani, F. (1998). Feature extraction by burst-like spike patterns in multiple sensory maps. Journal of Neuroscience, 18(6), 2283–2300.
Mills, S. L., Xia, X. B., Hoshi, H., Firth, S. I., Rice, M. E., Frishman, L. J., et al. (2007). Dopaminergic modulation of tracer coupling in a ganglion-amacrine cell network. Visual Neuroscience, 24(4), 593–608.
Pillow, J. W., Shlens, J., Paninski, L., Sher, A., Litke, A. M., Chichilnisky, E. J., et al. (2008). Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature, 454(7207), 995–999.
Reid, R. C., Victor, J. D., & Shapley, R. M. (1997). The use of m-sequences in the analysis of visual neurons: Linear receptive field properties. Visual Neuroscience, 14(6), 1015–1027.
Reinagel, P., Godwin, D., Sherman, S. M., & Koch, C. (1999). Encoding of visual information by LGN bursts. Journal of Neurophysiology, 81(5), 2558–2569.
Reinagel, P., & Reid, R. C. (2000). Temporal coding of visual information in the thalamus. Journal of Neuroscience, 20(14), 5392–5400.
Ribelayga, C., Cao, Y., & Mangel, S. C. (2008). The circadian clock in the retina controls rod-cone coupling. Neuron, 59(5), 790–801.
Schnitzer, M. J., & Meister, M. (2003). Multineuronal firing patterns in the signal from eye to brain. Neuron, 37(3), 499–511.
Strong, S. P., de Ruyter van Steveninck, R. R., Bialek, W., & Koberle, R. (1998). On the application of information theory to neural spike trains. Pac Symp Biocomput, 621–632.
Swadlow, H. A., & Gusev, A. G. (2001). The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nature Neuroscience, 4(4), 402–408. doi:10.1038/86054.
Teich, M. C., Turcott, R. G., & Siegel, R. M. (1996). Temporal correlation in cat striate-cortex neural spike trains. Engineering in Medicine and Biology Magazine, IEEE, 15(5), 79–87.
Usrey, W. M., Reppas, J. B., & Reid, R. C. (1999). Specificity and strength of retinogeniculate connections. Journal of Neurophysiology, 82(6), 3527–3540.
Wang, G. L., Zhou, Y., Chen, A. H., Zhang, P. M., & Liang, P. J. (2006). A robust method for spike sorting with automatic overlap decomposition. IEEE Transactions on Biomedical Engineering, 53(6), 1195–1198. doi:10.1109/TBME.2006.873397.
Witkovsky, P. (2004). Dopamine and retinal function. Documenta Ophthalmologica, 108(1), 17–40.
Witkovsky, P., & Dearry, A. (1991). Functional roles of dopamine in the vertebrate retina. Progress in Retinal Research, 11, 247–292.
Witkovsky, P., Nicholson, C., Rice, M. E., Bohmaker, K., & Meller, E. (1993). Extracellular dopamine concentration in the retina of the clawed frog, xenopus laevis. Proceedings of the National Academy of Sciences of the United States of America, 90(12), 5667–5671.
Zhang, P. M., Wu, J. Y., Zhou, Y., Liang, P. J., & Yuan, J. Q. (2004). Spike sorting based on automatic template reconstruction with a partial solution to the overlapping problem. Journal of Neuroscience Methods, 135(1–2), 55–65. doi:10.1016/j.jneumeth.2003.12.001.
Acknowledgments
This work was supported by the grants from the State Key Basic Research and Development Plan (No.2005CB724301) and National Foundations of Natural Science of China (No.61075108, No.60775034).
Conflicts of interest
No conflict of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Barry Richmond
Rights and permissions
About this article
Cite this article
Bu, JY., Li, H., Gong, HQ. et al. Gap junction permeability modulated by dopamine exerts effects on spatial and temporal correlation of retinal ganglion cells’ firing activities. J Comput Neurosci 36, 67–79 (2014). https://doi.org/10.1007/s10827-013-0469-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-013-0469-1