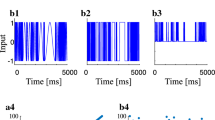
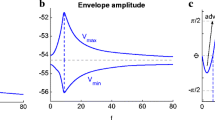
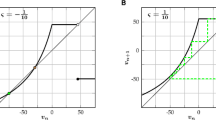
Abstract
Neuronal information transmission is fre- quency specific. In single cells, a band-pass like frequency preference can arise from the subthreshold dynamics of the membrane potential, shaped by properties of the cell’s membrane and its ionic channels. In these cases, a cell is termed resonant and its membrane impedance spectrum exhibits a peak at non-vanishing frequencies. Here, we show that this frequency selectivity of neuronal response amplitudes need not translate into a similar frequency selectivity of information transfer. In particular, neurons with resonant but linear subthreshold voltage dynamics (without threshold) do not show a resonance of information transfer at the level of subthreshold voltage; the corresponding coherence has low-pass characteristics. Interestingly, we find that when combined with nonlinearities, subthreshold resonances do shape the frequency dependence of coherence and the peak in the subthreshold impedance translates to a peak in the coherence function. In other words, the nonlinearity inherent to spike generation allows a subthreshold impedance resonance to shape a resonance of voltage-based information transfer. We demonstrate such nonlinearity-mediated band-pass filtering of information at frequencies close to the subthreshold impedance resonance in three different model systems: the resonate-and-fire model, the conductance-based Morris-Lecar model, and linear resonant dynamics combined with a simple static nonlinearity. In the spiking neuron models, the band-pass filtering is most pronounced for low firing rates and a high variability of interspike intervals, similar to the spiking statistics observed in vivo. We show that band-pass filtering is achieved by reducing information transfer over low-frequency components and, consequently, comes along with an overall reduction of information rate. Our work highlights the crucial role of nonlinearities for the frequency dependence of neuronal information transmission.
















Similar content being viewed by others
References
Alonso, A., & Llinas, R.R. (1989). Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer-II. Nature, 342, 175.
Badel, L., Lefort, S., Brette, R., Petersen, C.C.H., Gerstner, W., & Richardson, M.J.E. (2008). Dynamic I-V curves are reliable predictors of naturalistic pyramidal-neuron voltage traces. Journal of Neurophysiology, 99, 656.
Borst, A., & Theunissen, F. (1999). Information theory and neural coding. Nature Neuroscience, 2, 947.
Brunel, N., Hakim, V., & Richardson, M.J.E. (2003). Firing-rate resonance in a generalized integrate-and-fire neuron with subthreshold resonance. Phys Rev E, 67, 051,916.
Bussgang, J.J. (1952). Crosscorrelation functions of amplitudedistorted Gaussian signals. Research Lab. Electron.
Buzsáki, G., & Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science, 304(5679), 1926–1929.
Chacron, M.J., Doiron, B., Maler, L., Longtin, A., & Bastian, J. (2003). Non-classical receptive field mediates switch in a sensory neuron’s frequency tuning. Nature, 423, 77.
Droste, F., Schwalger, T., & Lindner, B. (2013). Interplay of two signals in a neuron with short-term synaptic plasticity. Frontiers in Computational Neuroscience, 7, 86.
Engel, T.A., Schimansky-Geier, L., Herz, A.V.M., Schreiber, S., & Erchova, I. (2008). Subthreshold membrane-potential resonances shape spike-train patterns in the entorhinal cortex. Journal of Neurophysiology, 100, 1576.
Erchova, I., Kreck, G., Heinemann, U., & Herz, A. (2004). Dynamics of rat entorhinal cortex layer II and III cells: characteristics of membrane potential resonance at rest predict oscillation properties near threshold. Journal of Neurophysiology, 560(Pt 1), 89.
Frigo, M., & Johnson, S.G. (2005). The design and implementation of FFTW3. Proceedings of the IEEE, 93(2), 216–231.
Gabbiani, F. (1996). Coding of time-varying signals in spike trains of linear and half-wave rectifying neurons. Network-Comp Neural, 7, 61.
Galassi, M., Davies, J., Theiler, J., Gough, B., Jungman, G., Alken, P., Booth, M., & Rossi, F. (2009). GNU scientific library reference manual-(v1. 12). Network Theory Ltd.
Gimbarzevsky, B., Miura, R.M., & Puil, E. (1984). Impedance profiles of peripheral and central neurons. Canadian Journal of Physiology and Pharmacology, 62(4), 460–462.
Gloveli, T., Schmitz, D., Empson, R.M., & Heinemann, U. (1997). Frequency-dependent information flow from the entorhinal cortex to the hippocampus. Journal of Neurophysiology, 78(6), 3444–3449.
Gutfreund, Y., Segev, I., & et al. (1995). Subthreshold oscillations and resonant frequency in guinea-pig cortical neurons: physiology and modelling. Journal of Neurophysiology, 483(Pt 3), 621– 640.
Gutkin, B.S., & Ermentrout, G.B. (1998). Dynamics of membrane excitability determine interspike interval variability: a link between spike generation mechanisms and cortical spike train statistics. Neural Computation, 10, 1047.
Hu, H., Vervaeke, K., & Storm, J.F. (2002). Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na + current in rat hippocampal pyramidal cells. The Journal of Physiology, 545(3), 783–805.
Hutcheon, B., & Yarom, Y. (2000). Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends in Neurosciences, 23(5), 216–222.
Hutcheon, B., Miura, R.M., & Puil, E. (1996). Models of subthreshold membrane resonance in neocortical neurons. Journal of Neurophysiology, 76(2), 698–714.
Izhikevich, E.M. (2001). Resonate-and-fire neurons. Neural Networks, 14, 883.
Izhikevich, E.M. (2007). Dynamical systems in neuroscience: the geometry of excitability and bursting. Cambridge: The MIT Press.
Kloeden, P., & Platen, E. (1992). Numerical Solution of Stochastic Differential Equations. Berlin: Springer.
Lindner, B. (2014). Low-pass filtering of information in the leaky integrate-and-fire neuron driven by white noise. In International Conference on Theory and Application in Nonlinear Dynamics (ICAND 2012) (pp. 249–258). Berlin: Springer.
Lindner, B., Schimansky-Geier, L., & Longtin, A. (2002). Maximizing spike train coherence or incoherence in the leaky integrate-and-fire model. Phys Rev E, 66, 031,916.
Lindner, B., García-Ojalvo, J., Neiman, A., & Schimansky-Geier, L. (2004). Effects of noise in excitable systems. Physics Reports, 392, 321.
Lindner, B., Gangloff, D., Longtin, A., & Lewis, J.E. (2009). Broadband coding with dynamic synapses. The Journal of Neuroscience, 29, 2076.
Mauro, A., Conti, F., Dodge, F., & Schor, R. (1970). Subthreshold behavior and phenomenological impedance of the squid giant axon. The Journal of General Physiology, 55(4), 497–523.
Middleton, J.W., Longtin, A., Benda, J., & Maler, L. (2009). Postsynaptic receptive field size and spike threshold determine encoding of high-frequency information via sensitivity to synchronous presynaptic activity. Journal of Neurophysiology, 101, 1160.
Morris, C., & Lecar, H. (1981). Voltage oscillations in the barnacle giant muscle fiber. Biophysical Journal, 35, 193.
Puil, E., Meiri, H., & Yarom, Y. (1994). Resonant behavior and frequency preferences of thalamic neurons. Journal of Neurophysiology, 71(2), 575–582.
Rau, F., Clemens, J., Naumov, V., Hennig, R.M., & Schreiber, S. (2015). Firing-rate resonances in the peripheral auditory system of the cricket, gryllus bimaculatus. Journal of Neurophysiology. submitted.
Rice, S.O. (1944). Mathematical analysis of random noise. Bell Syst Tech J, 23, 282.
Richardson, M.J.E., Brunel, N., & Hakim, V. (2003). From subthreshold to firing-rate resonance. Journal of Neurophysiology, 89, 2538.
Rieke, F., Bodnar, D., & Bialek, W. (1995). Naturalistic stimuli increase the rate and efficiency of information transmission by primary auditory afferents. Proceedings of the Biological Sciences, 262, 259.
Rieke, F., Warland, D., de Ruyter van Steveninck, R., & Bialek, W. (1996). Spikes: Exploring the neural code. Cambridge: MIT Press.
Rinzel, J., & Ermentrout, G.B. (1989). Analysis of neural excitability and oscillations. In Methods in Neuronal Modeling (pp. 135–169). Cambridge: MIT Press.
Risken, H. (1984). The Fokker-Planck equation. Berlin: Springer.
Roddey, J.C., Girish, B., & Miller, J.P. (2000). Assessing the performance of neural encoding models in the presence of noise. Journal of Computational Neuroscience, 8(2), 95–112.
Rosenbaum, R., Rubin, J., & Doiron, B. (2012). Short term synaptic depression imposes a frequency dependent filter on synaptic information transfer. PLoS Computational Biology, 8, e1002, 557.
Rotstein, H.G., & Nadim, F. (2014). Frequency preference in two-dimensional neural models: a linear analysis of the interaction between resonant and amplifying currents. Journal of Computational Neuroscience, 37(1), 9–28.
Sadeghi, S.G., Chacron, M.J., Taylor, M.C., & Cullen, K.E. (2007). Neural variability, detection thresholds, and information transmission in the vestibular system. The Journal of Neuroscience, 27(4), 771.
Schreiber, S. (2004). Frequency preference and reliability of signal integration: the role of intrinsic conductances. PhD thesis. Berlin: Humboldt University.
Schreiber, S., Erchova, I., Heinemann, U., & Herz, A.V. (2004). Subthreshold resonance explains the frequency-dependent integration of periodic as well as random stimuli in the entorhinal cortex. Journal of Neurophysiology, 92(1), 408–415.
Sharafi, N., Benda, J., & Lindner, B. (2013). Information filtering by synchronous spikes in a neural population. Journal of Computational Neuroscience, 34, 285.
Stein, B.E., & Stanford, T.R. (2008). Multisensory integration: current issues from the perspective of the single neuron. Nature Reviews Neuroscience, 9, 255.
Stein, R.B., Holden, A.V., & French, A.S. (1972). Frequency-response, coherence, and information capacity of 2 neuronal models. Biophysical Journal, 12, 295.
Vilela, R.D., & Lindner, B. (2009). A comparative study of three different integrate-and-fire neurons: spontaneous activity, dynamical response, and stimulus-induced correlation. Physical Review E, 80, 031,909.
Warland, D.K., Reinagel, P., & Meister, M. (1997). Decoding visual information from a population of retinal ganglion cells. Journal of Neurophysiology, 78, 2336.
Webb, B., Wessnitzer, J., Bush, S., Schul, J., Buchli, J., & Ijspeert, A. (2007). Resonant neurons and bushcricket behaviour. Journal of Comparative Physiology. A, 193(2), 285–288.
Acknowledgments
This work was supported by grants from the BMBF (01GQ0901,01GQ0972,01GQ1001A,01GQ1403) and Deutsche Forschungsgemeinschaft (SFB 618(B1)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Action Editor: Tim Gollisch
Appendix A
Appendix A
1.1 A.1 Gradual transitions from resonators to integrators
Here, we present our numerical scheme to differentiate gradually between resonators and integrators. This allows us to study the relationship between the power filter properties and the information filter properties of these neuron models. The question arises whether there is a relation between impedance and coherence quality. Therefore we have to vary the impedance quality with respect to certain constraints. These constraints are experimentally motivated and should hold under variation of the impedance quality
-
a)
resonance frequency is fixed (5 Hz, 9.5 Hz, 15 Hz),
-
b)
natural frequency is smaller than resonance frequency (see Fig. 10 in Erchova et al. 2004), here: \(\frac {f_{\mathrm nat}}{f_{\text {res}}}\approx 0.8\),
-
c)
band-with of the impedance resonance should be proportional to the resonance freq. (see Fig. 7B in Erchova et al. 2004)
-
d)
impedance quality should be varied in a systematic way,
We implemented a numerical scheme which solves a problem under these constraints. It turned out that the resistance of the inductor R L is a suitable parameter to vary the impedance quality with the above described constraints. Suitable means that the experimentally observed parameter set of the stellate cell should lie on our parameterization manifold (see Fig. 17).
Parameter sets yielding the same resonance frequency of the impedance in the linear, subthreshold dynamics. a Variation of impedance quality. By fixing the resonance frequency and changing the resistance of the inductor R L one can systematically vary the impedance quality. This way gradual transitions from resonators to integrators (including the physiological parameter set of the stellate cell, red data point) were obtained. b–d Lines of equal resonance frequency in subspaces of parameters of the subthreshold dynamics of the RF model (resistance R L versus damping factor, resistance R, and inductance L, respectively). e Impedance quality decreases with an increasing damping factor
Figure 17a–b shows how the increase of the resistance R L increases monotonically the impedance quality Q Z and changes the damping factor ζ, the membrane resistance R, and the inductance L.
We display in Fig. 17e the expected relationship between the damping factor ζ and the impedance quality: with increasing damping factor the impedance quality decreases. These approximately 75.000 parameter sets are then used to simulate the resonate-and-fire neuron model within different excitable regimes (variation of DC input I 0 at fixed D, D OU, V re s e t , τ abs) at different impedance resonance frequencies (see Figs. 9–11) in order to reveal the dependence of the filtering properties between power filtering, described by the impedance function and information filtering, described by the coherence function.
Rights and permissions
About this article
Cite this article
Blankenburg, S., Wu, W., Lindner, B. et al. Information filtering in resonant neurons. J Comput Neurosci 39, 349–370 (2015). https://doi.org/10.1007/s10827-015-0580-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-015-0580-6