Abstract
Simulating extracellular recordings of neuronal populations is an important and challenging task both for understanding the nature and relationships between extracellular field potentials at different scales, and for the validation of methodological tools for signal analysis such as spike detection and sorting algorithms. Detailed neuronal multicompartmental models with active or passive compartments are commonly used in this objective. Although using such realistic NEURON models could lead to realistic extracellular potentials, it may require a high computational burden making the simulation of large populations difficult without a workstation. We propose in this paper a novel method to simulate extracellular potentials of firing neurons, taking into account the NEURON geometry and the relative positions of the electrodes. The simulator takes the form of a linear geometry based filter that models the shape of an action potential by taking into account its generation in the cell body / axon hillock and its propagation along the axon. The validity of the approach for different NEURON morphologies is assessed. We demonstrate that our method is able to reproduce realistic extracellular action potentials in a given range of axon/dendrites surface ratio, with a time-efficient computational burden.















Similar content being viewed by others
Notes
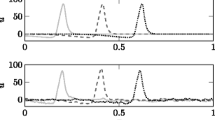
This simplifying assumption lacks in reproducing the intrinsic dendritic filtering shown in e.g., Lindén et al. (2010), as it will be discussed further in the Results section.
In other words, the dipolar moment at time t will be defined as j(t) = Ca(rk+ 1 −rk)Ik(t).
The same reasoning could be applied for (rk + 1 −rk) in Eq. 5 and Ca coefficient.
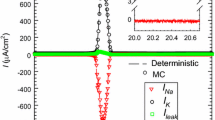
It is well known that a single compartment NEURON can not generate any extracellular potential because the Kirchhoff’s current law is not respected – the net transmembrane current must necessarily be equal to zero. The simplest NEURON model able to generate an LFP signature is then a two-compartment model where the membrane current enter the NEURON at one compartment and leaves at the other compartment.
A similar figure comparing performances of 1μ m and 2μ m diameter axons can be found in the Supplementary Material.
These values correspond in fact to τk equal to 21, respectively 12 samples, at a sampling frequency of 106Hz. These values are the median speeds over the optimal speed values for all configurations having a given axon diameter (for example, 0.45 is the medians of optimal v for all BS models with an axon of 2μ m diameter).
Note that this would allow to create signals for training or evaluating spike sorting algorithms (Lewicki 1998; Rey et al. 2015). Recall that these algorithms are based on distinct features of the EAPs (amplitude, width... etc), which our simulator is able to reproduce for varying positions. Note that supplementary variability could be in principle obtained by varying also the parameters of the HH model.
Only the part due to the EAP, no synaptic currents were taken into account, see Aussel et al. (2019) for preliminary results on the relative contributions of both synaptic and EAP currents to the extracellular potential.
When a single HH compartment is simulated for all 5000 neurons.
References
Archie, K.A., & Mel, B.W. (2000). A model for intradendritic computation of binocular disparity. Nature Neuroscience, 3(1), 54.
Aussel, A., Buhry, L., Tyvaert, L., Ranta, R. (2018). A detailed anatomical and mathematical model of the hippocampal formation for the generation of sharp-wave ripples and theta-nested gamma oscillations. Journal of Computational Neuroscience, 45(3), 207–221. https://doi.org/10.1007/s10827-018-0704-x.
Aussel, A., Tran, H., Buhry, L., Le Cam, S., Maillard, L., Colnat-Coulbois, S., Louis-Dorr, V., Ranta, R. (2019). Extracellular synaptic and action potential signatures in the hippocampal formation: a modelling study. In 28th Annual Computational Neuroscience Meeting, CNS’2019, Barcelona.
Barry, J.M. (2015). Axonal activity in vivo: technical considerations and implications for the exploration of neural circuits in freely moving animals. Frontiers in Neuroscience, 9, 153.
Bédard, C., & Destexhe, A. (2011). A generalized theory for current-source density analysis in brain tissue. Physical Review. E, Statistical, Nonlinear, and Soft Matter Physics, 84, 041909. arXiv:1101.1094v3.
Bieler, M., Sieben, K., Cichon, N., Schildt, S., Röder, B., Hanganu-Opatz, I.L. (2017). Rate and temporal coding convey multisensory information in primary sensory cortices. eNeuro, 4(2), ENEURO–0037.
Blanche, T.J., Spacek, M.A., Hetke, J.F., Swindale, N.V. (2005). Polytrodes: high-density silicon electrode arrays for large-scale multiunit recording. Journal of Neurophysiology, 93(5), 2987–3000.
Brette, R., & Destexhe, A. (2012). Handbook of neural activity measurement. Cambridge: Cambridge University Press.
Buccino, A.P., Kuchta, M., Jæger, K.H., Ness, T.V., Berthet, P., Mardal, K.A., Cauwenberghs, G., Tveito, A. (2019). How does the presence of neural probes affect extracellular potentials?. Journal of Neural Engineering, 16(2), 026030.
Buzsáki, G. (2004). Large-scale recording of neuronal ensembles. Nature Neuroscience, 7(5), 446.
Buzsáki, G., Anastassiou, C.A., Koch, C. (2012). The origin of extracellular fields and currents – EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience, 13(6), 407.
Camuñas-Mesa, L.A., & Quiroga, R.Q. (2013). A detailed and fast model of extracellular recordings. Neural Computation, 25(5), 1191–1212.
Chelaru, M.I., & Jog, M.S. (2005). Spike source localization with tetrodes. Journal of Neuroscience Methods, 142(2), 305–315.
Destexhe, A., Contreras, D., Steriade, M. (1999). Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. Journal of Neuroscience, 19(11), 4595–4608.
Dura-Bernal, S., Suter, B.A., Gleeson, P., Cantarelli, M., Quintana, A., Rodriguez, F., Kedziora, D.J., Chadderdon, G.L., Kerr, C.C., Neymotin, S.A., McDougal, R.A., Hines, M., Shepherd, G.M., Lytton, W.W. (2019). NetPyNE, a tool for data-driven multiscale modeling of brain circuits. eLife, 8, e44494. https://doi.org/10.7554/eLife.44494.
Einevoll, G.T., Kayser, C., Logothetis, N.K., Panzeri, S. (2013a). Modelling and analysis of local field potentials for studying the function of cortical circuits. Nature Reviews Neuroscience, 14(11), 770.
Einevoll, G.T., Linden, H., Tetzlaff, T., Leski, S., Pettersen, K.H. (2013b). Local field potentials. Principles of Neural Coding, 37.
Fiala, J.C., & Harris, K.M. (1999). Dendrite structure. Dendrites, 2, 1–11.
Gerstner, W., & Kistler, W.M. (2002). Spiking neuron models: Single neurons, populations, plasticity. Cambridge: Cambridge University Press.
Gold, C., Henze, D. A., Koch, C., Buzsaki, G. (2006). On the origin of the extracellular action potential waveform: a modeling study. Journal of Neurophysiology, 95(5), 3113–3128.
Gold, C., Henze, D.A., Koch, C. (2007). Using extracellular action potential recordings to constrain compartmental models. Journal of Computational Neuroscience, 1(23), 39–58.
Gomes, J.M., Bėdard, C., Valtcheva, S., Nelson, M., Khokhlova, V., Pouget, P., Venance, L., Bal, T., Destexhe, A. (2016). Intracellular Impedance Measurements Reveal Non-ohmic Properties of the Extracellular Medium around Neurons. Biophysical Journal, 110 (1), 234–46.
Goto, T., Hatanaka, R., Ogawa, T., Sumiyoshi, A., Riera, J., Kawashima, R. (2010). An evaluation of the conductivity profile in the somatosensory barrel cortex of wistar rats. Journal of Neurophysiology, 104(6), 3388–3412.
Hagen, E., Ness, T. V., Khosrowshahi, A., Sørensen, C., Fyhn, M., Hafting, T., Franke, F., Einevoll, G.T. (2015). Visapy: a python tool for biophysics-based generation of virtual spiking activity for evaluation of spike-sorting algorithms. Journal of Neuroscience Methods, 245, 182–204.
Hagen, E., Dahmen, D., Stavrinou, M.L., Lindén, H., Tetzlaff, T., van Albada, S.J., Grün, S., Diesmann, M., Einevoll, G.T. (2016). Hybrid scheme for modeling local field potentials from point-neuron networks. Cerebral Cortex, 1–36.
Hines, M.L., & Carnevale, N.T. (1997). The NEURON simulation environment. Neural Computation, 9(6), 1179–1209.
Hodgkin, A.L., & Huxley, A.F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of Physiology, 117(4), 500– 544.
Holt, G.R., & Koch, C. (1999). Electrical interactions via the extracellular potential near cell bodies. Journal of Computational Neuroscience, 6(2), 169–184.
Horowitz, A., Barazany, D., Tavor, I., Bernstein, M., Yovel, G., Assaf, Y. (2015). In vivo correlation between axon diameter and conduction velocity in the human brain. Brain Structure and Function, 220 (3), 1777–1788.
Kajikawa, Y., & Schroeder, C.E. (2011). How local is the local field potential? Neuron, 72(5), 847–858.
Kole, M.H., Ilschner, S.U., Kampa, B.M., Williams, S.R., Ruben, P.C., Stuart, G.J. (2008). Action potential generation requires a high sodium channel density in the axon initial segment. Nature Neuroscience, 11(2), 178.
Kress, G.J., & Mennerick, S. (2009). Action potential initiation and propagation: upstream influences on neurotransmission. Neuroscience, 158(1), 211–222.
Łėski, S., Lindén, H., Tetzlaff, T., Pettersen, K.H., Einevoll, G.T. (2013). Frequency dependence of signal power and spatial reach of the local field potential. PLos Computational Biology, 9(7), e1003137.
Lewandowska, M.K., Bakkum, D.J., Rompani, S.B., Hierlemann, A. (2015). Recording large extracellular spikes in microchannels along many axonal sites from individual neurons. Plos One, 10(3), e0118514.
Lewicki, M.S. (1994). Bayesian modeling and classification of neural signals. Neural Computation, 6(5), 1005–1030.
Lewicki, M.S. (1998). A review of methods for spike sorting: the detection and classification of neural action potentials. Network: Computation in Neural Systems, 9(4), R53–R78.
Lindén, H., Pettersen, K.H., Einevoll, G.T. (2010). Intrinsic dendritic filtering gives low-pass power spectra of local field potentials. Journal of Computational Neuroscience, 29(3), 423– 444.
Lindén, H., Tetzlaff, T., Potjans, T.C., Pettersen, K.H., Grün, S., Diesmann, M., Einevoll, G.T. (2011). Modeling the spatial reach of the LFP. Neuron, 72(5), 859–872.
Lindén, H., Hagen, E., Leski, S., Norheim, E.S., Pettersen, K.H., Einevoll, G.T. (2014). Lfpy: a tool for biophysical simulation of extracellular potentials generated by detailed model neurons. Frontiers in Neuroinformatics, 7, 41.
Logothethis, N., Kayser, C., Oeltermann, A. (2007). In vivo measurement of cortical impedance spectrum in monkeys: Implications for signal propagation. Neuron, 55, 809–823.
Mainen, Z.F., & Sejnowski, T.J. (1996). Influence of dendritic structure on firing pattern in model neocortical neurons. Nature, 382(6589), 363.
Martinez, J., Pedreira, C., Ison, M.J., Quiroga, R. Q. (2009). Realistic simulation of extracellular recordings. Journal of Neuroscience Methods, 184(2), 285–293.
Mazzoni, A., Lindén, H., Cuntz, H., Lansner, A., Panzeri, S., Einevoll, G.T. (2015). Computing the local field potential (LFP) from integrate-and-fire network models. PLos Computational Biology, 11(12), e1004584.
Mechler, F., & Victor, J.D. (2012). Dipole characterization of single neurons from their extracellular action potentials. Journal of Computational Neuroscience, 32(1), 73–100.
Mikelberg, F.S., Drance, S.M., Schulzer, M., Yidegiligne, H.M., Weis, M.M. (1989). The normal human optic nerve: axon count and axon diameter distribution. Ophthalmology, 96(9), 1325–1328.
Mitzdorf, U. (1985). Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiological Reviews, 65(1), 37–100.
Mondragón-González, S.L., & Burguière, E. (2017). Bio-inspired benchmark generator for extracellular multi-unit recordings. Scientific Reports, 7, 43253.
Ness, T.V., Chintaluri, C., Potworowski, J., ŁėSki, S., Głȧbska, H., Wójcik, D.K., Einevoll, G.T. (2015). Modelling and analysis of electrical potentials recorded in microelectrode arrays (meas). Neuroinformatics, 13(4), 403–426.
Nunez, P., & Srinivasan, R. (2006). Electric fields of the brain, 2nd edn. New York: Oxford University Press.
Parasuram, H., Nair, B., D’Angelo, E., Hines, M., Naldi, G., Diwakar, S. (2016). Computational modeling of single neuron extracellular electric potentials and network local field potentials using LFPsim. Frontiers in Computational Neuroscience, 10, 65.
Pesaran, B., Vinck, M., Einevoll, G.T., Sirota, A., Fries, P., Siegel, M., Truccolo, W., Schroeder, C.E., Srinivasan, R. (2018). Investigating large-scale brain dynamics using field potential recordings: analysis and interpretation. Nature Neuroscience, 21(7), 903–919.
Pettersen, K.H., & Einevoll, G.T. (2008). Amplitude variability and extracellular low-pass filtering of neuronal spikes. Biophysical Journal, 94(3), 784–802.
Pettersen, K.H., Hagen, E., Einevoll, G.T. (2008). Estimation of population firing rates and current source densities from laminar electrode recordings. Journal of Computational Neuroscience, 24(3), 291–313.
Pettersen, K.H., Lindén, H., Tetzlaff, T., Einevoll, G.T. (2011). The ball and stick neuron model accounts both for microscopic and macroscopic power laws. BMC Neuroscience, 12(1), P91.
Pettersen, H., Dale, A.M., Einevoll, G.T. (2012). Extracellular spikes and current-source density. handbook of neural activity measurements,. romain brette and a destexhe.
Pettersen, K.H., Lindén, H., Tetzlaff, T., Einevoll, G.T. (2014). Power laws from linear neuronal cable theory: power spectral densities of the soma potential, soma membrane current and single-neuron contribution to the eeg. PLos Computational Biology, 10(11), e1003928.
Peyrache, A., Dehghani, N., Eskandar, E.N., Madsen, J.R., Anderson, W.S., Donoghue, J.A., Hochberg, L.R., Halgren, E., Cash, S.S., Destexhe, A. (2012). Spatiotemporal dynamics of neocortical excitation and inhibition during human sleep. Proceedings of the National Academy of Sciences, 109(5), 1731–1736.
Rall, W., & Shepherd, G.M. (1968). Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. Journal of Neurophysiology, 31(6), 884–915.
Ranta, R., Le Cam, S., Tyvaert, L., Louis-Dorr, V. (2017). Assesing human brain impedance using simultaneous surface and intracerebral recordings. Neuroscience, 343, 411–422.
Rey, H.G., Pedreira, C., Quiroga, R.Q. (2015). Past, present and future of spike sorting techniques. Brain Research Bulletin, 119, 106–117.
Ritchie, J.M. (1982). On the relation between fibre diameter and conduction velocity in myelinated nerve fibres. Proceedings of the Royal Society of London B, 217(1206), 29–35.
Robbins, A.A., Fox, S.E., Holmes, G.L., Scott, R.C., Barry, J.M. (2013). Short duration waveforms recorded extracellularly from freely moving rats are representative of axonal activity. Frontiers in Neural Circuits, 7, 181.
Teleńczuk, M., Brette, R., Destexhe, A., Teleńczuk, B. (2018). Contribution of the axon initial segment to action potentials recorded extracellularly. eNeuro, ENEURO–0068.
Thorbergsson, P.T., Garwicz, M., Schouenborg, J., Johansson, A.J. (2012). Computationally efficient simulation of extracellular recordings with multielectrode arrays. Journal of Neuroscience Methods, 1(211), 133–144.
Tomsett, R.J., Ainsworth, M., Thiele, A., Sanayei, M., Chen, X., Gieselmann, M.A., Whittington, M.A., Cunningham, M.O., Kaiser, M. (2015). Virtual Electrode Recording Tool for EXtracellular potentials (VERTEX): comparing multi-electrode recordings from simulated and biological mammalian cortical tissue. Brain Structure and Function, 220(4), 2333–2353.
Toth, E., Fabo, D., Entz, L., Ulbert, I., Eross, L. (2016). Intracranial neuronal ensemble recordings and analysis in epilepsy. Journal of Neuroscience Methods, 260, 261–269.
Traub, R.D., Bibbig, A., LeBeau, F.E., Buhl, E.H., Whittington, M.A. (2004). Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annual Review of Neuroscience, 27, 247–278.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Action Editor: Gaute T. Einevoll
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tran, H., Ranta, R., Le Cam, S. et al. Fast simulation of extracellular action potential signatures based on a morphological filtering approximation. J Comput Neurosci 48, 27–46 (2020). https://doi.org/10.1007/s10827-019-00735-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-019-00735-3