Abstract
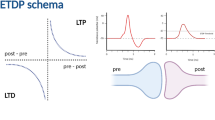
Hebbian plasticity means that if the firing of two neurons is correlated, then their connection is strengthened. Conversely, uncorrelated firing causes a decrease in synaptic strength. Spike-timing-dependent plasticity (STDP) represents one instantiation of Hebbian plasticity. Under STDP, synaptic changes depend on the relative timing of the pre- and post-synaptic firing. By inducing pre- and post-synaptic firing at different relative times the STDP curves of many neurons have been determined, and it has been found that there are different curves for different neuron types or synaptic sites. Biophysically, strengthening (long-term potentiation, LTP) or weakening (long-term depression, LTD) of glutamatergic synapses depends on the post-synaptic influx of calcium (Ca2+): weak influx leads to LTD, while strong, transient influx causes LTP. The voltage-dependent NMDA receptors are the main source of Ca2+ influx, but they will only open if a post-synaptic depolarisation coincides with pre-synaptic neurotransmitter release. Here we present a computational mechanism for Ca2+-dependent plasticity in which the interplay between the pre-synaptic neurotransmitter release and the post-synaptic membrane potential leads to distinct Ca2+ time-courses, which in turn lead to the change in synaptic strength. It is shown that the model complies with classic STDP results, as well as with results obtained with triplets of spikes. Furthermore, the model is capable of displaying different shapes of STDP curves, as observed in different experimental studies.









Similar content being viewed by others
References
Abarbanel, H.D., Gibb, L., Huerta, R., Rabinovich, M.I. (2003). Biophysical model of synaptic plasticity dynamics. Biological Cybernetics, 89(3), 214–226.
Abbott, L.F., & Nelson, S.B. (2000). Synaptic plasticity: taming the beast. Nature Neuroscience, 3(11s), 1178.
Badoual, M., Zou, Q., Davison, A.P., Rudolph, M., Bal, T., Frégnac, Y., Destexhe, A. (2006). Biophysical and phenomenological models of multiple spike interactions in spike-timing dependent plasticity. International Journal of Neural Systems, 16(02), 79–97.
Bi, G.-q., & Poo, M.-m. (1998). Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. Journal of Neuroscience, 18(24), 10464–10472.
Cai, Y., Gavornik, J.P., Cooper, L.N., Yeung, L.C., Shouval, H.Z. (2007). Effect of stochastic synaptic and dendritic dynamics on synaptic plasticity in visual cortex and hippocampus. Journal of Neurophysiology, 97(1), 375–386.
Carlson, K.D., & Giordano, N. (2011). Interplay of the magnitude and time-course of postsynaptic ca 2+ concentration in producing spike timing-dependent plasticity. Journal of Computational Neuroscience, 30(3), 747–758.
Clarke, R.J., & Johnson, J.W. (2006). Nmda receptor nr2 subunit dependence of the slow component of magnesium unblock. Journal of Neuroscience, 26(21), 5825–5834.
Colbran, R.J. (2004). Protein phosphatases and calcium/calmodulin-dependent protein kinase II-dependent synaptic plasticity. Journal of Neuroscience, 24(39), 8404–8409.
Dayan, P., & Abbott, L.F. (2001). Theoretical neuroscience Vol. 806. Cambridge: MIT Press.
DeMaria, C.D., Soong, T.W., Alseikhan, B.A., Alvania, R.S., Yue, D.T. (2001). Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature, 411(6836), 484.
Destexhe, A., Mainen, Z.F., Sejnowski, T.J. (1994a). An efficient method for computing synaptic conductances based on a kinetic model of receptor binding. Neural Computation, 6(1), 14–18.
Destexhe, A., Mainen, Z.F., Sejnowski, T.J. (1994b). Synthesis of models for excitable membranes, synaptic transmission and neuromodulation using a common kinetic formalism. Journal of Computational Neuroscience, 1(3), 195–230.
Destexhe, A., Mainen, Z.F., Sejnowski, T.J. (1998). Kinetic models of synaptic transmission. Methods in Neuronal Modeling, 2, 1–25.
Feldman, D.E. (2012). The spike-timing dependence of plasticity. Neuron, 75(4), 556–571.
Fino, E., Deniau, J.-M., Venance, L. (2008). Cell-specific spike-timing-dependent plasticity in GABAergic and cholinergic interneurons in corticostriatal rat brain slices. The Journal of Physiology, 586(1), 265–282.
Froemke, R.C., & Dan, Y. (2002). Spike-timing-dependent synaptic modification induced by natural spike trains. Nature, 416(6879), 433.
Froemke, R.C., Poo, M.-m., Dan, Y. (2005). Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature, 434(7030), 221.
Froemke, R.C., Tsay, I.A., Raad, M., Long, J.D., Dan, Y. (2006). Contribution of individual spikes in burst-induced long-term synaptic modification. Journal of Neurophysiology, 95(3), 1620–1629.
Gerkin, R.C., Lau, P.-M., Nauen, D.W., Wang, Y.T., Bi, G.-Q. (2007). Modular competition driven by NMDA receptor subtypes in spike-timing-dependent plasticity. Journal of Neurophysiology, 97(4), 2851–2862.
Gerkin, R.C., Bi, G.-Q., Rubin, J.E. (2010). A phenomenological calcium-based model of STDP. In Hippocampal microcircuits (pp. 571–591): Springer.
Gerstner, W., Kempter, R., van Hemmen, J.L., Wagner, H. (1996). A neuronal learning rule for sub-millisecond temporal coding. Nature, 383(6595), 76.
Graupner, M., & Brunel, N. (2007). STDP in a bistable synapse model based on CaMKII and associated signaling pathways. PLoS Computational Biology, 3(11), e221.
Graupner, M., & Brunel, N. (2010). Mechanisms of induction and maintenance of spike-timing dependent plasticity in biophysical synapse models. Frontiers in Computational Neuroscience, 4, 136.
Hebb, D. (1949). The organization of behavior. New York: Wiley.
Holmes, W.R. (2000). Models of calmodulin trapping and cam kinase ii activation in a dendritic spine. Journal of Computational Neuroscience, 8(1), 65–86.
Holthoff, K., Zecevic, D., Konnerth, A. (2010). Rapid time course of action potentials in spines and remote dendrites of mouse visual cortex neurons. The Journal of Physiology, 588(7), 1085–1096.
Jahr, C.E., & Stevens, C.F. (1990). Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. Journal of Neuroscience, 10(9), 3178–3182.
Kampa, B.M., Clements, J., Jonas, P., Stuart, G.J. (2004). Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity. The Journal of Physiology, 556(2), 337–345.
Karmarkar, U.R., & Buonomano, D.V. (2002). A model of spike-timing dependent plasticity: one or two coincidence detectors? Journal of Neurophysiology, 88(1), 507–513.
Kubota, S., & Kitajima, T. (2008). A model for synaptic development regulated by nmda receptor subunit expression. Journal of Computational Neuroscience, 24(1), 1–20.
Lester, R.A., Clements, J.D., Westbrook, G.L., Jahr, C.E. (1990). Channel kinetics determine the time course of nmda receptor-mediated synaptic currents. Nature, 346(6284), 565.
Letzkus, J.J., Kampa, B.M., Stuart, G.J. (2006). Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. Journal of Neuroscience, 26(41), 10420–10429.
Lisman, J. (1989). A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proceedings of the National Academy of Sciences, 86(23), 9574–9578.
Lisman, J. (2017). Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Philosophical Transactions of the Royal Society B, 372(1715), 20160260.
Lisman, J., Yasuda, R., Raghavachari, S. (2012). Mechanisms of CaMKII action in long-term potentiation. Nature Reviews Neuroscience, 13(3), 169.
London, M., & Häusser, M. (2005). Dendritic computation. Annual Review of Neuroscience, 28, 503–532.
Lu, J.-t., Li, C.-y., Zhao, J.-P., Poo, M.-m., Zhang, X.-h. (2007). Spike-timing-dependent plasticity of neocortical excitatory synapses on inhibitory interneurons depends on target cell type. Journal of Neuroscience, 27 (36), 9711–9720.
Malenka, R.C., & Bear, M.F. (2004). LTP and LTD: an embarrassment of riches. Neuron, 44(1), 5–21.
Malenka, R.C., Kauer, J.A., Perkel, D.J., Mauk, M.D., Kelly, P.T., Nicoll, R.A., Waxham, M.N. (1989). An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature, 340(6234), 554.
Malenka, R.C., Kauer, J.A., Zucker, R.S., Nicoll, R.A. (1988). Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science, 242(4875), 81–84.
Malinow, R., & Malenka, R.C. (2002). AMPA receptor trafficking and synaptic plasticity. Annual Review of Neuroscience, 25(1), 103–126.
Markram, H., Helm, P.J., Sakmann, B. (1995). Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. The Journal of Physiology, 485(1), 1–20.
Markram, H., Lübke, J., Frotscher, M., Sakmann, B. (1997). Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science, 275(5297), 213–215.
Mizuno, T., Kanazawa, I., Sakurai, M. (2001). Differential induction of ltp and ltd is not determined solely by instantaneous calcium concentration: an essential involvement of a temporal factor. European Journal of Neuroscience, 14(4), 701–708.
Nevian, T., & Sakmann, B. (2006). Spine Ca2+ signaling in spike-timing-dependent plasticity. Journal of Neuroscience, 26(43), 11001–11013.
Pfister, J.-P., & Gerstner, W. (2006). Triplets of spikes in a model of spike timing-dependent plasticity. Journal of Neuroscience, 26(38), 9673–9682.
Qian, A., Buller, A.L., Johnson, J.W. (2005). Nr2 subunit-dependence of nmda receptor channel block by external mg2+. The Journal of Physiology, 562(2), 319–331.
Rozsa, B., Zelles, T., Vizi, E.S., Lendvai, B. (2004). Distance-dependent scaling of calcium transients evoked by backpropagating spikes and synaptic activity in dendrites of hippocampal interneurons. Journal of Neuroscience, 24(3), 661–670.
Rubin, J.E., Gerkin, R.C., Bi, G.-Q., Chow, C.C. (2005). Calcium time course as a signal for spike-timing-dependent plasticity. Journal of Neurophysiology, 93(5), 2600–2613.
Sabatini, B.L., Oertner, T.G., Svoboda, K. (2002). The life cycle of ca2+ ions in dendritic spines. Neuron, 33(3), 439–452.
Shouval, H.Z., & Kalantzis, G. (2005). Stochastic properties of synaptic transmission affect the shape of spike time-dependent plasticity curves. Journal of Neurophysiology, 93(2), 1069–1073.
Shouval, H.Z., Bear, M.F., Cooper, L.N. (2002). A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proceedings of the National Academy of Sciences, 99(16), 10831–10836.
Sjöström, P.J., & Häusser, M. (2006). A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron, 51(2), 227–238.
Sjöström, P.J., & Nelson, S.B. (2002). Spike timing, calcium signals and synaptic plasticity. Current Opinion in Neurobiology, 12(3), 305–314.
Song, S., Miller, K.D., Abbott, L.F. (2000). Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nature Neuroscience, 3(9), 919.
Sourdet, V., & Debanne, D. (1999). The role of dendritic filtering in associative long-term synaptic plasticity. Learning & Memory, 6(5), 422–447.
Tzounopoulos, T., Kim, Y., Oertel, D., Trussell, L.O. (2004). Cell-specific, spike timing–dependent plasticities in the dorsal cochlear nucleus. Nature Neuroscience, 7(7), 719.
Urakubo, H., Honda, M., Froemke, R.C., Kuroda, S. (2008). Requirement of an allosteric kinetics of NMDA receptors for spike timing-dependent plasticity. Journal of Neuroscience, 28(13), 3310–3323.
Vargas-Caballero, M., & Robinson, H.P. (2003). A slow fraction of Mg2+ unblock of NMDA receptors limits their contribution to spike generation in cortical pyramidal neurons. Journal of Neurophysiology, 89(5), 2778–2783.
Wang, H.-X., Gerkin, R.C., Nauen, D.W., Bi, G.-Q. (2005). Coactivation and timing-dependent integration of synaptic potentiation and depression. Nature Neuroscience, 8(2), 187.
Waters, J., Schaefer, A., Sakmann, B. (2005). Backpropagating action potentials in neurones: measurement, mechanisms and potential functions. Progress in Biophysics and Molecular Biology, 87(1), 145–170.
Yang, S.-N., Tang, Y.-G., Zucker, R.S. (1999). Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. Journal of Neurophysiology, 81(2), 781–787.
Zenke, F., Agnes, E.J., Gerstner, W. (2015). Diverse synaptic plasticity mechanisms orchestrated to form and retrieve memories in spiking neural networks. Nature Communications, 6, 6922.
Acknowledgments
MSK acknowledges support from the grant PGC2018-099506-B-I00 from the Spanish Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest
Additional information
Action Editor: Albert Compte
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: Table of parameters & values used
Appendix B: Comparison of saturated and unsaturated equations
In this appendix the effects of adding or removing certain saturations of the proposed models are investigated. As visible in Figs. 11 and 12 the removal of the saturations of Eq. (9) and (12), respectively, does not affect the results in a qualitative manner. The absolute values of the resulting Af and As signals differ, but the relative values between the different spike timings stay preserved as in the case of the main text (Fig. 10).
However removing the saturation in Eq. (13) leads to qualitatively different results, as shown in Fig. 13a. After inspection it was found that the main difference between the saturised and un-saturised model are the maximal and mean levels reached by the Af signal for different relative spike timings. Figure 13b shows the maximal levels of the Af signal for different spike timings and shows that the un-saturised model (grey line) has highter maximal values especially for large spiking intervals than the saturised model (black line). The values of the normalised mean Af values, as shown in Fig. 13c, show that in the saturised model (black line) the basic shape of the STDP window is shown by the mean Af values, whereas the un-saturised model (grey line) shows a very different shape.
Appendix C: Equivalence of NMDA receptor product and difference of exponentials models
In the main text the variable governing the open-probability of the post-synaptic NMDA receptors is modelled as a product of a slow and a fast variable (see Eqs. (5) & (6)). More conventionally, a difference of exponentials is used to model the NMDA receptor open probability, which is fitted to follow the opening probability of kinetic synaptic transmission models as proposed by Destexhe et al. (1994a, b, 1998). The difference of exponentials, as used by Dayan and Abbott (2001), is given as:
in which B is a normalising factor and τ1 > τ2 are time constants. The rise time of the variable Ps is given by: \(\tau _{rise} = \frac {\tau _{1} \tau _{2}}{\tau _{1} - \tau _{2}}\) and the decay time is given by τ1.
The variable used in this article is the product of a slow and a fast variable Pnmda = P+P−. P+ and P− are governed by the differential equations (5) and (6), respectively. Integrating these equations, assuming a pre-synaptic pulse at time t = 0 gives:
Leading to:
Then, by using the parameters from the main text (τ+ = 1.5ms and τ− = 152ms), one finds that the decay time of the difference of exponentials is τ1 = τ− = 152ms, and the rise time τrise ≈ 1.5ms. Thus it is shown that the product of a slow and fast variable and the difference of exponentials models for synaptic opening are equivalent in this case. Figure 14 compares the results of a numerical simulation of the kinetic scheme for the NMDA synaptic transmission, as described in Destexhe et al. (1998), with the model for Pnmda used in this manuscript (Eqs. (5) and (6)).
Appendix D: Comparison of NMDA receptor opening simulations
In the presented model the NMDA recptor opening gnmda (4) is goverened by the NMDA receptor open probability Pnmda (Eqs. (5) and (6)), which is related to the binding of glutamate to the receptor, and the Mg2+ unblocking \(\widetilde {G}\), which is modelled as a low-passed version of the direct Mg2+ unblocking as given by Eq. (7) (Jahr and Stevens 1990; Dayan and Abbott 2001). Using a low-passed version of the Mg2+ unblocking is motivated by the observation that the unblock is not instantaneous (Vargas-Caballero and Robinson 2003; Kampa et al. 2004) and that the specific channel sub-unit make-up can alter the Mg2+ unblocking time-course (Vargas-Caballero and Robinson 2003; Qian et al. 2005; Clarke and Johnson 2006). In their paper, Kampa et al. (2004) provide a kinetic model that captures the non-instantaneous effect of the Mg2+ unblocking as they find in their data. This appendix provides a comparison between the kinetic model and Eqs. (4) through (8).
Simulations of both the kinetic model of Kampa et al. (2004) and Eqs. (4)–(8) have been done using one pre-post and one post-pre spike pairing, the same as during one run on the STDP induction protocol, with an inter-spike interval of 10ms. Figure 15 shows the resulting NMDA receptor opening following a pre-post pairing, Fig. 16 shows the NMDA receptor opening following a post-pre pairing. The black lines show the NMDA receptor opening as predicted by the Kampa et al. (2004) kinetic model, the NMDA receptor opening used in this paper is given by the grey lines. The Pnmda rise and fall time constants of Eqs. (5) and (6), respectively, have been altered in order to obtain a better fit with the kineticmodel (τ+ = 2ms, τ− = 105ms).
Comparison of the kinetic model of NMDA receptor opening (black line) and the NMDA receptor opening used in the current paper (grey line) for a single pre-post pairing (tpre − tpost = − 10ms). In this simulation τ+ = 2ms (5) and τ− = 105ms (6). Pre-synaptic timing (glutamate release) is indicated by the vertical line. Post-synaptic activity occurs at the arrow
Comparison of the kinetic model of NMDA receptor opening (black line) and the NMDA receptor opening used in the current paper (solid line) for a single post-pre pairing (tpre − tpost = 10ms). In this simulation τ+ = 2ms (5) and τ− = 105ms (6). Pre-synaptic timing (glutamate release) is indicated by the vertical line. Post-synaptic activity occurs at the arrow
The results show large agreement in NMDA receptor opening between the kinetic model and Eqs. (4)–(8). The opening time-courses in the pre-post spike pairing case, shown in Fig. 15, show that the model used in this paper follows a decay time-course with a slight change in the decay rate around t ≈ 120ms in Fig. 15, due to the different interacting terms that lead to the NMDA receptor opening. However the two methods are in agreement on the large-scale shape and magnitude of the NMDA receptor opening. Figure 16 shows the results of both methods following a post-pre spike pairing. The peak magnitude of the NMDA receptor opening given by Eqs. (4)–(8) is slightly lower than that of the kinetic model, however, the models agree on the large scale shape of the NMDA receptor opening time-course.
Rights and permissions
About this article
Cite this article
Houben, A.M., Keil, M.S. A calcium-influx-dependent plasticity model exhibiting multiple STDP curves. J Comput Neurosci 48, 65–84 (2020). https://doi.org/10.1007/s10827-019-00737-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-019-00737-1