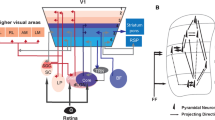
Abstract
Between the onset of the critical period of mouse primary visual cortex and eye opening at postnatal day 14 is a complex process and that is vital for the cognitive function of vision. The onset of the critical period of mouse primary visual cortex involves changes of the intrinsic firing property of each neuron and short term plasticity of synapses. In order to investigate the functional role of each factor in regulating the circuit firing activity during the critical period plasticity, we adopted the Markram’s model for short term plasticity and Wilson’s model for intrinsic neuron firing activity, and construct a microcircuit for mouse visual cortex layer IV based on the connection probabilities from experimental results. Our results indicate that, during CP development, the most critical factors that regulate the firing pattern of microcircuit is the short term plasticity of the synapse from PC to PV and SST interneurons, which upregulates the PV interneuron firing and produces new balance between excitation and inhibition; the intrinsic firing activity of PC and PV during development downregulates the firing frequency of the circuits. In addition, we have investigated the function of feedforward excitatory thalamic-cortical projection to PC and PV interneuron during CP, and found that neural firing activity largely depends on the TC input and the results are similar to the local circuit with minor differences. We conclude that the short term plasticity development during critical period plays a crucial role in regulating the circuit behavior.











Similar content being viewed by others
References
Beierlein, M. (2003). Two dynamically distinct inhibitory networks in layer 4 of the neocortex. Journal of Neurophysiology, 90(5), 2987.
Cardin, J. A., Carlen, M., Meletis, K., Knoblich, U., Zhang, F., Deisseroth, K., Tsai, L. H., & Moore, C. I. (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature, 459(7247), 663–667.
Cisneros-Franco, J. M., & de Villers-Sidani, É. (2019). Reactivation of critical period plasticity in adult auditory cortex through chemogenetic silencing of parvalbumin-positive interneurons. Proceedings of the National Academy of Sciences, 116(52), 26329–26331. https://doi.org/10.1073/pnas.1913227117.
Cruikshank, S. J., Urabe, H., Nurmikko, A. V., & Connors, B. W. (2010). Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron, 65(2), 230–245.
Davis, M. F., Figueroa Velez, D. X., Guevarra, R. P., Yang, M. C., Habeeb, M., Carathedathu, M. C., & Gandhi, S. P. (2015). Inhibitory neuron transplantation into adult visual cortex creates a new critical period that rescues impaired vision. Neuron, 86(4), 1055–1066.
Espinosa, J. S., & Stryker, M. P. (2012). Development and plasticity of the primary visual cortex. Neuron, 75(2), 230–249.
Fagiolini, M., Fritschy, J. M., Low, K., Mohler, H., Rudolph, U., & Hensch, T. K. (2004). Specific GABAA circuits for visual cortical plasticity. Science, 303(5664), 1681–1683.
Gonchar, Y., Wang, Q., & Burkhalter, A. (2007). Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Frontiers in Neuroanatomy, 1, 3.
Gu, Y., Huang, S., Chang, M. C., Worley, P., Kirkwood, A., & Quinlan, E. M. (2013). Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron, 79(2), 335–346.
Gutierrez, C., Cox, C. L., Rinzel, J., & Sherman, S. M. (2001). Dynamics of low-threshold spike activation in relay neurons of the cat lateral geniculate nucleus. The Journal of Neuroscience, 21(3), 1022–1032.
Hanover, J. L., Huang, Z. J., Tonegawa, S., & Stryker, M. P. (1999). Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. Journal of Neuroscience, 19(22), RC40.
Hensch, T. K., & Fagiolini, M. (2005). Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Progress in Brain Research, 147, 115–124.
Hensch, T. K., Fagiolini, M., Mataga, N., Stryker, M. P., Baekkeskov, S., & Kash, S. F. (1998). Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science, 282(5393), 1504–1508.
Huang, Z. J., Kirkwood, A., Pizzorusso, T., Porciatti, V., Morales, B., Bear, M. F., Maffei, L., & Tonegawa, S. (1999). BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell, 98(6), 739–755.
Jang, H. J., Chung, H., Rowland, J. M., Richards, B. A., Kohl, M. M., & Kwag, J. (2020). Distinct roles of parvalbumin and somatostatin interneurons in gating the synchronization of spike times in the neocortex. Science Advances, 6(47), eaay5333.
Levelt, C. N., & Hubener, M. (2012). Critical-period plasticity in the visual cortex. Annual Review of Neuroscience, 35, 309–330.
Kelsom, C., & Lu, W. (2013). Development and specification of GABAergic cortical interneurons. Cell & Bioscience, 3, 19.
Khibnik, L. A., Cho, K. K., & Bear, M. F. (2010). Relative contribution of feedforward excitatory connections to expression of ocular dominance plasticity in layer 4 of visual cortex. Neuron, 66(4), 493–500.
Ko, H., Cossell, L., Baragli, C., Antolik, J., Clopath, C., Hofer, S. B., & Mrsic-Flogel, T. D. (2013). The emergence of functional microcircuits in visual cortex. Nature, 496(7443), 96–100.
Kuhlman, S. J., Lu, J., Lazarus, M. S., & Huang, Z. J. (2010). Maturation of GABAergic inhibition promotes strengthening of temporally coherent inputs among convergent pathways. PLoS Computational Biology, 6(6), e1000797.
Kuhlman, S. J., Tring, E., & Trachtenberg, J. T. (2011). Fast-spiking interneurons have an initial orientation bias that is lost with vision. Nature Neuroscience, 14(9), 1121–1123.
Liu, B. H., Wu, G. K., Arbuckle, R., Tao, H. W., & Zhang, L. I. (2007). Defining cortical frequency tuning with recurrent excitatory circuitry. Nature Neuroscience, 10(12), 1594–1600.
Markram, H., Yun, W., & Tsodyks, M. (1998). Differential signaling via the same axon of neocortical pyramidal neurons. Proceedings of the National Academy of Sciences of the United States of America, 95(9), 5323–5328.
Miao, Q., Yao, L., Rasch, M., Ye, Q., Li, X., & Zhang, X. (2016). Selective maturation of temporal dynamics of intracortical excitatory transmission at the critical period onset. Cell Reports, 16(6), 1677–1689.
Markram, H., Lübke, J., Frotscher, M., & Sakmann, B. (1997). Regulation of synaptic efficacy by coincidence of postsynaptic aps and epsps. Science, 275(5297), 213–215.
Markram, H., Toledo-Rodriguez, M., Wang, Y., Gupta, A., Silberberg, G., & Wu, C. (2004). Interneurons of the neocortical inhibitory system. Nature Reviews Neuroscience, 5(10), 793–807.
Miao, Q. (2014). Circuit Mechanisms underlying the early developmental regulation of visual cortical plasticity, PhD thesis.
Oswald, A. M., & Reyes, A. D. (2011). Development of inhibitory timescales in auditory cortex. Cerebral Cortex, 21(6), 1351–1361.
Pan, N. C., Fang, A., Shen, C., Sun, L., Wu, Q., & Wang, X. (2019). Early excitatory activity-dependent maturation of somatostatin interneurons in cortical layer 2/3 of mice. Cerebral Cortex, 29(10), 4107–4118.
Ramoa, A. S., & Sur, M. (1996). Short-term synaptic plasticity in the visual cortex during development. Cerebral Cortex, 6(4), 640–646.
Rozov, A., & Burnashev, N. (1999). Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature, 401(6753), 594–598.
Sakurai, I., Kubota, S., & Niwano, M. (2014). The onset and closure of critical period plasticity regulated by feedforward inhibition. Neurocomputing, 143, 261–268.
Sale, A., Berardi, N., Spolidoro, M., Baroncelli, L., & Maffei, L. (2010). GABAergic inhibition in visual cortical plasticity. Frontiers in Cellular Neuroscience, 4, 10.
Sohal, V. S., Zhang, F., Yizhar, O., & Deiseeroth, K. (2009). Parvalbulmin neurons and gamma rhythms enhance cortical circuit performance. Nature, 459(7247), 698–702.
Traub, R. D., Kopell, N., Bibbig, A., Buhl, E. H., LeBeau, F. E., & Whittington, M. A. (2001). Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. The Journal of Neuroscience, 21(23), 9478–9486.
Tsodyks, M. V., & Markram, H. (1997). The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proceedings of the National Academy of Sciences, 94(2), 719–723.
Tuncdemir, S. N., Wamsley, B., Stam, F. J., Osakada, F., Goulding, M., Callaway, E. M., Rudy, B., & Fishell, C. (2016). Early somatostatin interneuron connectivity mediates the maturation of deep layer cortical circuits. Neuron, 89, 521–535.
Trappenberg, T. P. (2010). Fundamentals of computational neuroscience. Oxford University Press, 2nd edition.
Tremblay, R., Lee, S., & Rudy, B. (2016). GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron, 91, 260–292. https://doi.org/10.1016/j.neuron.2016.06.033.
Tsodyks, M., Pawelzik, K., & Markram, H. (1998). Neural networks with dynamic synapses. Neural Computation, 10(4), 821–835.
Virginia, G. M., Kelly, J. G., & Hawken, M. J. (2019). Major feedforward thalamic input into layer 4C of primary visual cortex in primate. Cerebral Cortex, 29(1), 134–149. https://doi.org/10.1093/cercor/bhx311
Wang, X. J., Rinzel, J., & Rogawski, M. A. (1991). A model of the t-type calcium current and the low-threshold spike in thalamic neurons. Journal of Neurophysiology, 66(3), 839–850.
Wilson, H. R. (1999). Simplified dynamics of human and mammalian neocortical neurons. Journal of Theoretical Biology, 200(4), 375–388.
Zucker, R. S., & Regehr, W. G. (2002). Short-term synaptic plasticity. Annual Review of Physiology, 64, 355–405.
Acknowledgements
We thank Dr. Yao (Li, Yao) for helpful discussions. This research is supported by National Key R&D Program of China (No. 2019 YFA0709503), Beijing high-level discipline construction project–cognitive neuroscience, and China National Science Foundation (No. 31601145).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors declared that they have no conflicts of interest to this work.
Additional information
Action Editor: Ken Miller
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Critical Period (CP) development is a specific period during early development after eye opening for mouse at postnatal day P14-P20 until the critical period onset (P20-21). The plasticity in mouse primary visual cortex layer IV during CP development involves changes of the intrinsic neuronal firing property and synaptic short term plasticity. We have investigated the functional roles of each factor in regulating the firing pattern of the microcircuits of visual cortex IV during the critical period. For local circuits, the short term plasticity changes of PC output synapses during CP increases PC and PV firing activity intensively, while the intrinsic neuronal firing properties of PC and PV interneuron down regulates the circuit’s firing activity. The local circuit switches from PC and SST firing patterns before CP to PC and PV firing patterns after CP onset. For global circuits with thalamus cell injection, neural firing activity is basically similar to the local circuit with minor differences, indicating that the short term plasticity development during critical period plays a crucial role in regulating the circuit behavior.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Li, Y. Deciphering functional roles of synaptic plasticity and intrinsic neural firing in developing mouse visual cortex layer IV microcircuit. J Comput Neurosci 51, 23–42 (2023). https://doi.org/10.1007/s10827-022-00823-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-022-00823-x