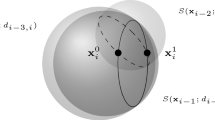
Abstract
Proteins are important molecules that are widely studied in biology. Since their three-dimensional conformations can give clues about their function, an optimal methodology for the identification of such conformations has been researched for many years. Experiments of Nuclear Magnetic Resonance (NMR) are able to estimate distances between some pairs of atoms forming the protein, and the problem of identifying the possible conformations satisfying the available distance constraints is known in the scientific literature as the Molecular Distance Geometry Problem (MDGP). When some particular assumptions are satisfied, MDGP instances can be discretized, and solved by employing an ad-hoc algorithm, named the interval Branch & Prune. When dealing with molecules such as proteins, whose chemical structure is known, a priori information can be exploited for generating atomic orderings that allow for the discretization. In previous publications, we presented a handcrafted order for the protein backbones. In this work, we propose 20 new orders for the 20 side chains that can be present in proteins. Computational experiments on artificial and real instances from NMR show the usefulness of the proposed orders.








Similar content being viewed by others
References
Berman, H., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T., Weissig, H., Shindyalov, I., Bourne, P.: The protein data bank. Nucleic Acid Res. 28, 235–242 (2000)
Costa, V., Mucherino, A., Lavor, C., Carvalho, L.M., Maculan, N.: On Suitable Orders for Discretizing Molecular Distance Geometry Problems related to Protein Side Chains. In: IEEE Conference Proceedings, Federated Conference on Computer Science and Information Systems (FedCSIS12), pp. 397–402, Wroclaw, Poland, 2012. Workshop on Computational, Optimization (WCO12)
Costa, V., Mucherino, A., Carvalho, L.M., Maculan, N.: On the Discretization of \(i\)DMDGP instances regarding Protein Side Chains with rings. In: Proceedings of Distance Geometry and Applications (DGA13), Manaus, Amazonas, Brazil, pp. 99–102 (2013)
Crippen, G., Havel, T.: Distance Geometry and Molecular Conformation. Wiley, New York (1988)
Donald, B.: Algorithms in Structural Molecular Biology. MIT Press, Boston (2011)
Grishaev, A., Bax, A.: An empirical backbone-backbone hydrogen-bonding potential in proteins and its applications to nmr structure refinement and validation. J. Am. Chem. Soc. 126, 7281–7292 (2004)
Havel, T.: Distance geometry. In: Grant, D.M., Harris, R.K. (eds.) Encyclopedia of Nuclear Magnetic Resonance, pp. 1701–1710. Wiley, New York (1995)
Honig, B., Nicholls, A.: Classical electrostatics in biology and chemistry. Science 268, 1144–1149 (1995)
Lavor, C., Lee, J., Lee-St. John, A., Liberti, L., Mucherino, A., Sviridenko, M.: Discretization orders for distance geometry problems. Optim. Lett. 6(4), 783–796 (2012)
Lavor, C., Liberti, L., Maculan, N., Mucherino, A.: The discretizable molecular distance geometry problem. Comput. Optim. Appl. 52, 115–146 (2012)
Lavor, C., Liberti, L., Mucherino, A.: The interval Branch-and-Prune algorithm for the discretizable molecular distance geometry problem with inexact distances. J. Global Optim. 56, 855–871 (2013)
Liberti, L., Lavor, C., Maculan, N., Mucherino, A.: Euclidean distance geometry and applications. SIAM Rev. 56(1) (2014)
Liberti, L., Lavor, C., Mucherino, A., Maculan, N.: Molecular distance geometry methods: from continuous to discrete. Int. Trans. Oper. Res. 18, 33–51 (2010)
Mucherino, A., Lavor, C., Liberti, L., Maculan, N. (eds.): Distance Geometry: Theory, Methods and Applications, 410 pp. Springer, Berlin (2013)
Mucherino, A., Lavor, C., Malliavin, T., Liberti, L., Nilges, M., Maculan, N.: Influence of pruning devices on the solution of molecular distance geometry problems. In: Pardalos, P.M., Rebennack, S. (eds.) Lecture Notes in Computer Science 6630, Proceedings of the 10th International Symposium on Experimental Algorithms (SEA11), Crete, Greece, pp. 206–217 (2011)
Nilges, M., Clore, G., Gronenborn, A.: Determination of three-dimensional structures of proteins from interproton distance data by hybrid distance geometry-dynamical simulated annealing calculations. Fed. Eur. Biochem. Soc. 229, 317–324 (1988)
Rocchia, W., Alexov, E., Honig, B.: Extending the applicability of the nonlinear Poisson–Boltzmann equation: multiple dielectric constants and multivalent ions. J. Phys. Chem. B 105, 6507–6514 (2001)
Sallaume, S., Martins, S., Ochi, L., Gramacho, W., Lavor, C., Liberti, L.: A discrete search algorithm for finding the structure of protein backbones and side chains. Int. J. Bioinf. Res. Appl. 9, 261–270 (2013)
Saxe, J.: Embeddability of weighted graphs in \(k\)-space is strongly NP-hard. In: Proceedings of 17th Allerton Conference in Communications, Control and Computing, pp. 480–489 (1979)
Schlick, T.: Molecular Modelling and Simulation: An Interdisciplinary Guide. Springer, New York (2002)
Souza, M., Lavor, C., Muritiba, A., Maculan, N.: Solving the molecular distance geometry problem with inaccurate distance data. BMC Bioinf. 14(Suppl 9), S7 (2013)
Souza, M., Xavier, A., Lavor, C., Maculan, N.: Hyperbolic smoothing and penalty techniques applied to molecular structure determination. Oper. Res. Lett. 39, 461–465 (2011)
Acknowledgments
We are thankful to Thérèse Malliavin and Douglas Gonçalves for the fruitful comments on this paper, as well as to the anonymous referees. We also wish to thank CAPES, that funded a 4-month visit to Rennes for Virginia Costa: part of this work was performed during such a visit. We are also thankful to the French Embassy in São Paulo and UNICAMP (which funded a 2-month chaire in UNICAMP for Antonio Mucherino), to the Brazilian research agencies FAPESP and CNPq, and to the French National Research Agency (ANR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costa, V., Mucherino, A., Lavor, C. et al. Discretization orders for protein side chains. J Glob Optim 60, 333–349 (2014). https://doi.org/10.1007/s10898-013-0135-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10898-013-0135-1