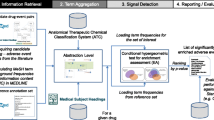
Abstract
We report on our research in using literature-based discovery (LBD) to provide pharmacological and/or pharmacogenomic explanations for reported adverse drug effects. The goal of LBD is to generate novel and potentially useful hypotheses by analyzing the scientific literature and optionally some additional resources. Our assumption is that drugs have effects on some genes or proteins and that these genes or proteins are associated with the observed adverse effects. Therefore, by using LBD we try to find genes or proteins that link the drugs with the reported adverse effects. These genes or proteins can be used to provide insight into the processes causing the adverse effects. Initial results show that our method has the potential to assist in explaining reported adverse drug effects.
Similar content being viewed by others
References
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y., Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10:796–803, 2013. doi:10.7150/ijms.6048.
Avillach, P., Dufour, J.-C., Diallo, G., et al., Design and validation of an automated method to detect known adverse drug reactions in MEDLINE: a contribution from the EU-ADR project. J. Am. Med. Inform. Assoc. 20:446–52, 2013. doi:10.1136/amiajnl-2012-001083.
Warrer, P., Hansen, E. H., Juhl-Jensen, L., and Aagaard, L., Using text-mining techniques in electronic patient records to identify ADRs from medicine use. Br. J. Clin. Pharmacol. 73:674–84, 2012. doi:10.1111/j.1365-2125.2011.04153.x.
Li, Y., Ryan, P. B., Wei, Y., and Friedman, C., A method to combine signals from spontaneous reporting systems and observational healthcare data to detect adverse drug reactions. Drug Saf. 38:895–908, 2015. doi:10.1007/s40264-015-0314-8.
Benton, A., Ungar, L., Hill, S., et al., Identifying potential adverse effects using the web: a new approach to medical hypothesis generation. J. Biomed. Inform. 44:989–96, 2011. doi:10.1016/j.jbi.2011.07.005.
Freifeld, C. C., Brownstein, J. S., Menone, C. M., et al., Digital drug safety surveillance: monitoring pharmaceutical products in twitter. Drug Saf. 37:343–350, 2014. doi:10.1007/s40264-014-0155-x.
Swanson, D. R., Fish oil, Raynaud’s syndrome, and undiscovered public knowledge. Perspect. Biol. Med. 30:7–18, 1986.
Hristovski, D., Rindflesch, T., and Peterlin, B., Using literature-based discovery to identify novel therapeutic approaches. Cardiovasc. Hematol. Agents Med. Chem. 11:14–24, 2013.
Hristovski, D., Kastrin, A., Peterlin, B., and Rindflesch, T. C., Combining Semantic Relations and DNA Microarray Data for Novel Hypotheses Generation. Link Lit. Inf. Knowl. Biol. 6004(Str):53–61, 2010. doi:10.1007/978-3-642-13131-8.
Hristovski, D., Dinevski, D., Kastrin, A., and Rindflesch, T. C., Biomedical question answering using semantic relations. BMC Bioinform. 16:6, 2015. doi:10.1186/s12859-014-0365-3.
Hristovski D., SemBT. http://sembt.mf.uni-lj.si. 2009.
Rindflesch, T. C., and Fiszman, M., The interaction of domain knowledge and linguistic structure in natural language processing: interpreting hypernymic propositions in biomedical text. J. Biomed. Inform. 36:462–77, 2003. doi:10.1016/j.jbi.2003.11.003.
Liverani, E., Leonardi, F., Castellani, L., et al., Asymptomatic and persistent elevation of pancreatic enzymes in an ulcerative colitis patient. Case Rep. Gastrointest. Med. 2013:415619, 2013. doi:10.1155/2013/415619.
Ventrucci, M., Pezzilli, R., Naldoni, P., et al., Serum pancreatic enzyme behavior during the course of acute pancreatitis. Pancreas 2:506–9, 1987.
Schmitz-Moormann, P., Comparative radiological and morphological study of the human pancreas. IV. acute necrotizing pancreatitis in man. Pathol. Res. Pract. 171:325–35, 1981. doi:10.1016/S0344-0338(81)80105-7.
Magos, L., Cikrt, M., and Snowden, R., The dependence of biliary methylmercury secretion on liver GSH and ligandin. Biochem. Pharmacol. 34:301–5, 1985.
Schoenberg, M. H., Büchler, M., Pietrzyk, C., et al., Lipid peroxidation and glutathione metabolism in chronic pancreatitis. Pancreas 10:36–43, 1995.
Akai, S., Hosomi, H., Minami, K., et al., Knock down of gamma-glutamylcysteine synthetase in rat causes acetaminophen-induced hepatotoxicity. J. Biol. Chem. 282:23996–4003, 2007. doi:10.1074/jbc.M702819200.
Kuhn, J. G., Pharmacology of irinotecan. Oncology (Williston Park) 12:39–42, 1998.
Xu, J.-M., Wang, Y., Ge, F.-J., et al., Severe irinotecan-induced toxicity in a patient with UGT1A1 28 and UGT1A1 6 polymorphisms. World J. Gastroenterol. 19:3899–903, 2013. doi:10.3748/wjg.v19.i24.3899.
Stock, J., Statin-associated muscle symptoms EAS Consensus Panel paper focuses on this neglected patient group. Atherosclerosis 242:346–50, 2015. doi:10.1016/j.atherosclerosis.2015.06.049.
Niemi, M., Transporter pharmacogenetics and statin toxicity. Clin. Pharmacol. Ther. 87:130–3, 2010. doi:10.1038/clpt.2009.197.
Schröder, J. P., Mau, W., Schumacher, S., and Zierz, S., Abnormal regulation of carnitine palmitoyltransferase in monozygotic twins as the cause of rhabdomyolysis. Dtsch. Med. Wochenschr. 115:337–9, 1990. doi:10.1055/s-2008-1065012.
Roglans, N., Sanguino, E., Peris, C., et al., Atorvastatin treatment induced peroxisome proliferator-activated receptor alpha expression and decreased plasma nonesterified fatty acids and liver triglyceride in fructose-fed rats. J. Pharmacol. Exp. Ther. 302:232–9, 2002.
Keverline, J. P., Recurrent rhabdomyolysis associated with influenza-like illness in a weight-lifter. J. Sports Med. Phys. Fitness 38:177–9, 1998.
Yang, S.-H., Choi, J.-S., and Choi, D.-H., Effects of HMG-CoA reductase inhibitors on the pharmacokinetics of losartan and its main metabolite EXP-3174 in rats: possible role of CYP3A4 and P-gp inhibition by HMG-CoA reductase inhibitors. Pharmacology 88:1–9, 2011. doi:10.1159/000328773.
Dopazo, C., Bilbao, I., Lázaro, J. L., et al., Severe rhabdomyolysis and acute renal failure secondary to concomitant use of simvastatin with rapamycin plus tacrolimus in liver transplant patient. Transplant. Proc. 41:1021–4, 2009. doi:10.1016/j.transproceed.2009.02.019.
Acknowledgments
This work was supported in part by the Intramural Research Program of the U.S. National Institutes of Health, National Library of Medicine. Authors would like to thank Celine Narjoz and Marie-Anne Loriot for suggesting the additional adverse drug reactions, which we used in this study. We are also grateful for the contribution of the medical students (Faculty of Medicine, University of Maribor) in the evaluation of the extracted relations.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Education & Training.
Rights and permissions
About this article
Cite this article
Hristovski, D., Kastrin, A., Dinevski, D. et al. Using Literature-Based Discovery to Explain Adverse Drug Effects. J Med Syst 40, 185 (2016). https://doi.org/10.1007/s10916-016-0544-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10916-016-0544-z