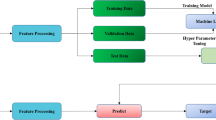
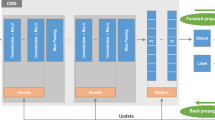
Abstract
A deep learning model was developed to identify osteoporosis from chest X-ray (CXR) features with high accuracy in internal and external validation. It has significant prognostic implications, identifying individuals at higher risk of all-cause mortality. This Artificial Intelligence (AI)-enabled CXR strategy may function as an early detection screening tool for osteoporosis. The aim of this study was to develop a deep learning model (DLM) to identify osteoporosis via CXR features and investigate the performance and clinical implications. This study collected 48,353 CXRs with the corresponding T score according to Dual energy X-ray Absorptiometry (DXA) from the academic medical center. Among these, 35,633 CXRs were used to identify CXR- Osteoporosis (CXR-OP). Another 12,720 CXRs were used to validate the performance, which was evaluated by the area under the receiver operating characteristic curve (AUC). Furthermore, CXR-OP was tested to assess the long-term risks of mortality, which were evaluated by Kaplan‒Meier survival analysis and the Cox proportional hazards model. The DLM utilizing CXR achieved AUCs of 0.930 and 0.892 during internal and external validation, respectively. The group that underwent DXA with CXR-OP had a higher risk of all-cause mortality (hazard ratio [HR] 2.59, 95% CI: 1.83–3.67), and those classified as CXR-OP in the group without DXA also had higher all-cause mortality (HR: 1.67, 95% CI: 1.61–1.72) in the internal validation set. The external validation set produced similar results. Our DLM uses CXRs for early detection of osteoporosis, aiding physicians to identify those at risk. It has significant prognostic implications, improving life quality and reducing mortality. AI-enabled CXR strategy may serve as a screening tool.






Similar content being viewed by others
Data availability
The data analyzed in this study is not publicly available due to privacy and security concerns. The data may be shared with a third party upon execution of data sharing agreement for reasonable requests, such requests should be addressed to WHF (e-mail: rumaf.fang@gmail.com) or DJT.
Abbreviations
- AI:
-
Artificial Intelligence
- AUC:
-
Area Under the Curve
- AMI:
-
Acute Myocardial Infarction
- Afib:
-
Atrial fibrillation
- BMD:
-
Bone Mineral Density
- CT:
-
Computed Tomography
- CXR:
-
Chest X-Ray
- CVD :
-
Cardiovascular Disease
- CV:
-
Cardiovascular
- CAD:
-
Coronary Artery Disease
- DXA:
-
Dual energy X-ray Absorptiometry
- DLM:
-
Deep Learning Model
- DICOM:
-
Digital Imaging and Communications in Medicine
- HR:
-
Hazard Ratio
- HF:
-
Heart failure
- ICD:
-
International Classification of Diseases
- NPV:
-
Negative Predictive Value
- OP:
-
Osteoporosis
- PPV:
-
Positive Predictive Value
- ROC:
-
Receiver Operating Characteristic
- STK:
-
Stroke
- Sens.:
-
Sensitivity
- Spec.:
-
Specificity
- WHO:
-
World Health Organization
References
Gharib, H., et al., Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract, 2010. 16(Suppl 1): p. 1-43.
Organization, W.H. WHO scientific group on the assessment of osteoporosis at primary health care level. in Summary meeting report. 2004.
Curry, S.J., et al., Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. Jama, 2018. 319(24): p. 2521-2531.
Shao, C.-J., et al., A nationwide seven-year trend of hip fractures in the elderly population of Taiwan. Bone, 2009. 44(1): p. 125-129.
Wooltorton, E., Osteoporosis treatment: raloxifene (Evista) and stroke mortality. Cmaj, 2006. 175(2): p. 147.
Bliuc, D., et al., Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. Jama, 2009. 301(5): p. 513-21.
Ganry, O., et al., Bone mass density and risk of breast cancer and survival in older women. Eur J Epidemiol, 2004. 19(8): p. 785-92.
Cai, S., et al., Bone mineral density and osteoporosis in relation to all-cause and cause-specific mortality in NHANES: A population-based cohort study. Bone, 2020. 141: p. 115597.
Yamamoto, N., et al., Deep learning for osteoporosis classification using hip radiographs and patient clinical covariates. Biomolecules, 2020. 10(11): p. 1534.
Mueller, D. and A. Gandjour, Cost‐effectiveness of using clinical risk factors with and without DXA for osteoporosis screening in postmenopausal women. Value in Health, 2009. 12(8): p. 1106-1117.
Orimo, H., et al., Japanese 2011 guidelines for prevention and treatment of osteoporosis—executive summary. Archives of osteoporosis, 2012. 7: p. 3-20.
Sedlak, C.A., M.O. Doheny, and S.L. Jones, Osteoporosis education programs: changing knowledge and behaviors. Public health nursing, 2000. 17(5): p. 398-402.
Sato, M., et al., Bone fractures and feeling at risk for osteoporosis among women in Japan: patient characteristics and outcomes in the National Health and Wellness Survey. Archives of Osteoporosis, 2014. 9: p. 1-9.
Compston, J.E., M.R. McClung, and W.D. Leslie, Osteoporosis. Lancet, 2019. 393(10169): p. 364-376.
Curtis, J.R., et al., Longitudinal trends in use of bone mass measurement among older americans, 1999-2005. J Bone Miner Res, 2008. 23(7): p. 1061-7.
Davis, S.R., et al., Simplifying screening for osteoporosis in Australian primary care: the Prospective Screening for Osteoporosis; Australian Primary Care Evaluation of Clinical Tests (PROSPECT) study. Menopause, 2011. 18(1): p. 53-9.
Yasaka, K., et al., Prediction of bone mineral density from computed tomography: application of deep learning with a convolutional neural network. European radiology, 2020. 30: p. 3549-3557.
Pickhardt, P.J., et al., Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Annals of internal medicine, 2013. 158(8): p. 588-595.
Krishnaraj, A., et al., Simulating dual-energy X-ray absorptiometry in CT using deep-learning segmentation cascade. Journal of the American College of Radiology, 2019. 16(10): p. 1473-1479.
Dagan, N., et al., Automated opportunistic osteoporotic fracture risk assessment using computed tomography scans to aid in FRAX underutilization. Nature medicine, 2020. 26(1): p. 77-82.
Benhamou, C.-L., et al., Fractal analysis of radiographic trabecular bone texture and bone mineral density: two complementary parameters related to osteoporotic fractures. Journal of bone and mineral research, 2001. 16(4): p. 697-704.
LeCun, Y., Y. Bengio, and G. Hinton, Deep learning. nature, 2015. 521(7553): p. 436–444.
Lindsey, R., et al., Deep neural network improves fracture detection by clinicians. Proceedings of the National Academy of Sciences, 2018. 115(45): p. 11591-11596.
He, K., et al. Delving deep into rectifiers: Surpassing human-level performance on imagenet classification. in Proceedings of the IEEE international conference on computer vision. 2015.
Gulshan, V., et al., Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. Jama, 2016. 316(22): p. 2402-2410.
Ardila, D., et al., End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nature medicine, 2019. 25(6): p. 954-961.
Smets, J., et al., Machine learning solutions for osteoporosis—a review. Journal of bone and mineral research, 2021. 36(5): p. 833-851.
Nguyen, T.P., et al., A novel approach for evaluating bone mineral density of hips based on Sobel gradient-based map of radiographs utilizing convolutional neural network. Computers in Biology and Medicine, 2021. 132: p. 104298.
Hsieh, C.-I., et al., Automated bone mineral density prediction and fracture risk assessment using plain radiographs via deep learning. Nature communications, 2021. 12(1): p. 5472.
Zhang, B., et al., Deep learning of lumbar spine X-ray for osteopenia and osteoporosis screening: A multicenter retrospective cohort study. Bone, 2020. 140: p. 115561.
Jang, M., et al., Opportunistic osteoporosis screening using chest radiographs with deep learning: Development and external validation with a cohort dataset. Journal of Bone and Mineral Research, 2022. 37(2): p. 369-377.
Sato, Y., et al., Deep Learning for Bone Mineral Density and T-Score Prediction from Chest X-rays: A Multicenter Study. Biomedicines, 2022. 10(9).
Chang, C.H., et al., Electrocardiogram-based heart age estimation by a deep learning model provides more information on the incidence of cardiovascular disorders. Front Cardiovasc Med, 2022. 9: p. 754909.
Liu, W.T., et al., A deep-learning algorithm-enhanced system integrating electrocardiograms and chest X-rays for diagnosing aortic dissection. Can J Cardiol, 2022. 38(2): p. 160-168.
Seok, H., et al., High prevalence of spine–femur bone mineral density discordance and comparison of vertebral fracture risk assessment using femoral neck and lumbar spine bone density in Korean patients. Journal of bone and mineral metabolism, 2014. 32: p. 405-410.
El Maghraoui, A., et al., Prevalence and risk factors of discordance in diagnosis of osteoporosis using spine and hip bone densitometry. Annals of the rheumatic diseases, 2007. 66(2): p. 271-272.
Kumar, D.A. and M. Anburajan, The role of hip and chest radiographs in osteoporotic evaluation among south Indian women population: a comparative scenario with DXA. Journal of endocrinological investigation, 2014. 37: p. 429-440.
Holcombe, S.A., et al., Measuring rib cortical bone thickness and cross section from CT. Med Image Anal, 2018. 49: p. 27-34.
Chen, H., et al., Age-related changes in trabecular and cortical bone microstructure. Int J Endocrinol, 2013. 2013: p. 213234.
Yao, W.J., et al., Differential Changes in Regional Bone Mineral Density in Healthy Chinese: Age-Related and Sex-Dependent. Calcified Tissue International, 2001. 68(6): p. 330-336.
Rajaei, A., et al., Correlating Whole-Body Bone Mineral Densitometry Measurements to Those From Local Anatomical Sites. Iran J Radiol, 2016. 13(1): p. e25609.
Sato, Y., et al., Deep learning for bone mineral density and T-score prediction from chest X-rays: A multicenter study. Biomedicines, 2022. 10(9): p. 2323.
Siris, E.S., et al., Bone mineral density thresholds for pharmacological intervention to prevent fractures. Archives of internal medicine, 2004. 164(10): p. 1108-1112.
Kanis, J.A., et al., European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis international, 2013. 24(1): p. 23-57.
Camacho, P.M., et al., American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocrine Practice, 2020. 26: p. 1-46.
Molarius, A. and S. Janson, Self-rated health, chronic diseases, and symptoms among middle-aged and elderly men and women. Journal of Clinical Epidemiology, 2002. 55(4): p. 364-370.
Hallberg, I., et al., Health-related quality of life after osteoporotic fractures. Osteoporosis International, 2004. 15: p. 834-841.
Hannan, M.T., et al., Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. Journal of Bone and Mineral Research, 2000. 15(4): p. 710-720.
De Laet, C.E. and H.A. Pols, Fractures in the elderly: epidemiology and demography. Best Practice & Research Clinical Endocrinology & Metabolism, 2000. 14(2): p. 171-179.
Berry, S.D., et al., Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc, 2010. 58(4): p. 783-7.
Kanis, J.A., et al., FRAX® and its applications to clinical practice. Bone, 2009. 44(5): p. 734-743.
Nguyen, N.D., et al., Development of a nomogram for individualizing hip fracture risk in men and women. Osteoporosis International, 2007. 18: p. 1109-1117.
Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser, 1994. 843: p. 1–129.
Funding
National Science and Technology Council,NSTC 112-2222-E-030 -002 -MY2,NSTC 112-2321-B-016-003,Ministry of Science and Technology,Taiwan,MOST110-2314-B-016-010-MY3.
Author information
Authors and Affiliations
Contributions
All authors participated in designing the study, generating hypotheses, interpreting the data, and critically reviewing the paper. DJT and WHF wrote the first draft, and CL, CSL, CCL, and CHW contributed substantially to writing subsequent versions. DJT designed and conducted statistical analyses with support from CL. All authors had full access to all the data in the study and accepted responsibility for the decision to submit for publication. DJT and WHF verified all the data used in this study. The corresponding author (WHF) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Ethics approval
The Tri-Service General Hospital, Taipei, Taiwan, conducted the ethical review of this study (IRB No. C202105049). The institutional review board agreed to waive individual consent since the data were collected retrospectively and analyzed on the intranet.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsai, DJ., Lin, C., Lin, CS. et al. Artificial Intelligence-enabled Chest X-ray Classifies Osteoporosis and Identifies Mortality Risk. J Med Syst 48, 12 (2024). https://doi.org/10.1007/s10916-023-02030-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10916-023-02030-2