Abstract
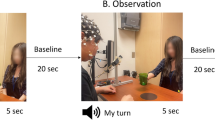
The left and right movements of a human or humanoid robot as an action observation stimulus will activate the human mirror neuron system (hMNS) to generate contralateral event-related desynchronization (ERD) suppression of the brain. The activation of hMNS response to action observation remains controversial for several reasons. Researches have found the influence of different speed factor for brain’s ERD suppression, but lack of exploring the influence of different speed discrepancy of left and right movements. To explore how is the discrepancy movements of left and right under different speeds influence ERD suppression, this paper invited six healthy subjects to participate action observation experiment under four different speeds (low, moderate, fast, and finalistic). Meanwhile, four different discrepancy speed ratios of movements are applied for such four different speeds to explore the influence of speed discrepancy on the left and right for the hMNS lateralized modulation effect. For the recorded electroencephalography (EEG) signals under different action observation stimulus, this paper selected the occipital and sensorimotor brain regions and used the convolutional neural network to classify EEG signals and measure the ERD suppression. Experimental results have shown that the action observation stimulus with different speed discrepancies improved the lateralized activity during low and moderate speeds, and significantly improved the lateralized activity than non-discrepancy during fast and finalistic speeds. We also analyzed the temporal, spectral, and classification characteristics for speed discrepancy, and discussed that the stimulus material design with speed discrepancy could greatly improve the lateralized modulation of ERD suppression. Action observation material with speed discrepancy of left and right movements could generate significant ERD suppression differences on lateralization of the brain, which could be used to build a more complex and robust brain-computer interface.









Similar content being viewed by others
References
Adcock J, Wise RG, Oxbury J et al (2003) Quantitative fmri assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage 18(2):423–438
Aflalo T, Kellis S, Klaes C, Lee B, Shi Y, Pejsa K, Shanfield K, Hayes-Jackson S, Aisen M, Heck C, Liu C, Andersen RA (2015) Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 348(6237):906–910
Ang KK, Chin ZY, Wang C, Guan C, Zhang H (2012) Filter bank common spatial pattern algorithm on bci competition iv datasets 2a and 2b. Front Neurosci 6:39
Aziz-Zadeh L, Maeda F, Zaidel E, Mazziotta J, Iacoboni M (2002) Lateralization in motor facilitation during action observation: a tms study. Exp Brain Res 144(1):127–131
Aziz-Zadeh L, Iacoboni M, Zaidel E, Wilson S, Mazziotta J (2004) Left hemisphere motor facilitation in response to manual action sounds. Eur J Neurosci 19(9):2609–2612
Aziz-Zadeh L, Koski L, Zaidel E, Mazziotta J, Iacoboni M (2006) Lateralization of the human mirror neuron system. J Neurosci 26(11):2964–2970
Aziz-Zadeh L, Iacoboni M, Zaidel E (2006) Hemispheric sensitivity to body stimuli in simple reaction time. Exp Brain Res 170(1):116–121
Aziz-Zadeh L, Liew SL, Dandekar F (2012) Exploring the neural correlates of visual creativity. Soc Cogn Affect Neurosci 8(4):475–480
Barham MP, Clark GM, Hayden MJ, Enticott PG, Conduit R, Lum JAG (2017) Acquiring research-grade erps on a shoestring budget: a comparison of a modified emotiv and commercial synamps eeg system. Psychophysiology 54(9):1393–1404
Belitski A, Farquhar J, Desain P (2011) P300 audio-visual speller. Journal of Neural Engineering 8(2):025022
Bentin S, McCarthy G, Wood CC (1985)Event-related potentials, lexical decision and semantic priming. Electroencephalogr Clin Neurophysiol 60(4):343–355
Bianco V, Di Russo F, Perri RL et al (2017) Different proactive and reactive action control in fencers’ and boxers’ brain. Neuroscience 343:260–268
Bianco V, Berchicci M, Perri RL et al (2017) The proactive self-control of actions: Time-course of underlying brain activities. NeuroImage 156:388–393
Bolognini N, Russo C, Edwards DJ (2016) The sensory side of poststroke motor rehabilitation. Restor Neurol Neurosci 34(4):571–586
Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ (2001) Action observation activates premotor and parietal areas in a somatotopic manner: an fmri study. Eur J Neurosci 13(2):400–404
Caligiore D, Mustile M, Spalletta G (2017) Action observation and motor imagery for rehabilitation in Parkinson’s disease: a systematic review and an integrative hypothesis. Neurosci Biobehav Rev 72:210–222
Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P (2004) Action observation and acquired motor skills: an fmri study with expert dancers. Cereb Cortex 15(8):1243–1249
Caspers S, Zilles K, Laird AR, Eickhoff SB (2010) Eickhoff.Ale meta-analysis of action observation and imitation in the human brain. Neuroimage 50(3):1148–1167
Claflin ES, Krishnan C, Khot SP (2015) Emerging treatments for motor rehabilitation after stroke. Neurohospitalist 5(2):77–88
Coll MP, Press C, Hobson H, Catmur C, Bird G (2017) Crossmodal classification of mu rhythm activity during action observation and execution suggests specificity to somatosensory features of actions. J Neurosci 37(24):5936–5947
Cristina LM, Matei D, Ignat B (2015) Mirror therapy enhances upper extremity motor recovery in stroke patients. Acta Neurol Belg 115(4):597–603
Di Dio C, Ardizzi M, Massaro D et al (2016) Human, nature, dynamism: the effects of content and movement perception on brain activations during the aesthetic judgment of representational paintings. Front Hum Neurosci 9:705
Donati AR, Shokur S, Morya E et al (2016) Longterm training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci Rep 6:30383
Donchin E, Spencer KM, Wijesinghe R (2000) The mental prosthesis: assessing the speed of a P300-based brain-computer interface. IEEE Trans Rehab Eng 8(2):174–179
Eaves DL, Riach M, Holmes PS, Wright DJ (2016) Motor imagery during action observation: a brief review of evidence, theory and future research opportunities. Front Neurosci 10:514
Ehrsson HH, Geyer S, Naito E (2003) Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol 90(5):3304–3316
El-Sourani N, Wurm MF, Trempler I et al (2018) Schubotz. Making sense of objects lying around: How contextual objects shape brain activity during action observation Neuroimage 167:429–437
Fadiga L, Fogassi L, Pavesi G, Rizzolatti G (1995) Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol 73(6):2608–2611
Fox NA, Bakermans-Kranenburg MJ, Yoo KH et al (2016) Assessing human mirror activity with eeg mu rhythm: A meta-analysis. Psychological Bulletin 142(3):291
Gallivan JP, Culham JC (2015) Neural coding within human brain areas involved in actions. Curr Opin Neurobiol 33:141–149
Gazzola V, Aziz-Zadeh L, Keysers C (2006) Empathy and the somatotopic auditory mirror system in humans. Curr Biol 16(18):1824–1829
Häberling IS, Corballis PM, Corballis MC (2016) Language, gesture, and handedness: Evidence for independent lateralized networks. Cortex 82:72–85
Hajipour Sardouie S, Shamsollahi MB (2012) Selection of efficient features for discrimination of hand movements from meg using a bci competition iv data set. Front Neurosci 6:42
Hardwick RM, Caspers S, Eickhoff SB, Swinnen SP (2018) Neural correlates of action: comparing meta-analyses of imagery, observation, and execuion. Neuroscience & Biobehavioral Reviews 94:31–44
Holmes PS, Wright DJ (2017) Motor cognition and neuroscience in sport psychology. Curr Opin Psychol 16:43–47
Ioffe S, Szegedy C (2015) Batch normalization: Accelerating deep network training by reducing internal covariate shift .arXiv preprint arXiv. 1502.03167
Kelly R, Mizelle J, Wheaton LA (2015) Distinctive laterality of neural networks supporting action understanding in left-and right-handed individuals: an eeg coherence study. Neuropsychologia 75:20–29
Kingma DP, Ba J (2014) Adam: A method for stochastic optimization. arXiv preprint arXiv 1412.6980
Koul A, Cavallo A, Cauda F, Costa T, Diano M, Pontil M, Becchio C (2018) Action observation areas represent intentions from subtle kinematic features. Cereb Cortex 28(7):2647–2654
Lange J, Pavlidou A, Schnitzler A (2015) Lateralized modulation of beta-band power in sensorimotor areas during action observation. Front Integr Neurosci 9:43
Lapergue B, Blanc R, Guedin P, Decroix JP, Labreuche J, Preda C, Bartolini B, Coskun O, Redjem H, Mazighi M, Bourdain F, Rodesch G, Piotin M (2016) A direct aspiration, first pass technique (adapt) versus stent retrievers for acute stroke therapy: an observational comparative study. Am J Neuroradiol 37(10):1860–1865
Lim H, Ku J (2018) A brain–computerinterface-based action observation game that enhances mu suppression. IEEE Trans Neural Syst Rehab Eng 26(12):2290–2296
Lingnau A, Downing PE (2015) The lateral occipitotemporal cortex in action. Trends Cogn Sci 19(5):268–277
Liu M, Wu W, Gu Z, Yu Z, Qi FF, Li Y (2018) Deep learning based on batch normalization for p300 signal detection. Neurocomputing 275:288–297
Lu N, Li T, Ren X, Miao H (2016) A deep learning scheme for motor imagery classification based on restricted boltzmann machines. IEEE Transactions on Neural Systems and Rehabilitation Engineering 25(6):566–576
Luo T, Lv J, Chao F, Zhou C (2018) Effect of different movement speed modes on human action observation: an eeg study. Front Neurosci 12:219
Luo T, Chao F, Zhou C (2018) Exploring spatial-frequency-sequential relationships for motor imagery classification with recurrent neural network. BMC Bioinformatics 19(1):344
M Mahsereci, L Balles, C Lassner et al (2017) Early stopping without a validation set. arXiv preprint arXiv, vol. 1703.09580.
Mäki-Marttunen V, Villarreal M, Leiguarda RC (2014) Lateralization of brain activity during motor planning of proximal and distal gestures. Behav Brain Res 272:226–237
Marangon M, Priftis K, Fedeli M et al (2014) Lateralization of motor cortex excitability in stroke patients during action observation: a tms study. BioMed Research International 2014
Marcroft C, Khan A, Embleton ND, Trenell M, Plötz T (2015) Movement recognition technology as a method of assessing spontaneous general movements in high risk infants. Front Neurol 5:284
Nouchine H, Joseph RM, Josh S et al (2006) Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex 16(9):1276–1282
Park EC, Hwangbo G (2015) The effects of action observation gait training on the static balance and walking ability of stroke patients. J Phys Ther Sci 27(2):341–344
Pattnaik S, Dash M, Sabut S (2016)Dwt-based feature extraction and classification for motor imaginary eeg signals. In: The 2016 international conference on Systems in Medicine and Biology (ICSMB). IEEE, Kharagpur, pp 186–201
Pereira J, Ofner P, Schwarz A, Sburlea AI, Müller-Putz GR (2017) Eeg neural correlates of goal-directed movement intention. Neuroimage 149:129–140
Pichiorri F, Morone G, Petti M, Toppi J, Pisotta I, Molinari M, Paolucci S, Inghilleri M, Astolfi L, Cincotti F, Mattia D (2015)Brain–computer interface boosts motor imagery practice during stroke recovery. Ann Neurol 77(5):851–865
Pineda JA (2008) Sensorimotor cortex as a critical component of an’extended’mirror neuron system: Does it solve the development, correspondence, and control problems in mirroring? Behavioral and Brain Functions 4(1):47
Pot E, Monceaux J, Gelin R et al (2009) Choregraphe: a graphical tool for humanoid robot programming. In: The 18th IEEE international symposium on robot and human interactive communication. IEEE, Roman, pp 46–51
Rakotomamonjy A, Guigue V (2008) Bci competition iii: dataset iiensemble of svms for bci p300 speller. IEEE Trans Biomed Eng 55(3):1147–1154
Ravi A, Beni NH, Manuel J et al (2020) Comparing user-dependent and user-independent training of CNN for SSVEP BCI. J Neural Eng 17(2):026028
Richard L, Charbonneau D (2009) An introduction to e-prime. Tutorials in Quantitative Methods for Psychology 5(2):68–76
Rizzolatti G, Craighero L (2004) The mirror-neuron system. Annu Rev Neurosci 27:169–192
Rizzolatti G, Sinigaglia C (2016) The mirror mechanism: a basic principle of brain function. Nature Reviews Neuroscience 17(12):757
Saitoh T, Zhou Z, Zhao G et al (2016) Concatenated frame image based cnn for visual speech recognition. In: The 13th proceedings of the Asian conference on computer vision. Springer, Taipei, pp 277–289
Schirrmeister RT, Springenberg JT, Fiederer LDJ, Glasstetter M, Eggensperger K, Tangermann M, Hutter F, Burgard W, Ball T (2017) Deep learning with convolutional neural networks for eeg decoding and visualization. Hum Brain Mapp 38(11):5391–5420
Schlögl A, Keinrath C, Zimmermann D, Scherer R, Leeb R, Pfurtscheller G (2007) A fully automated correction method of eog artifacts in eeg recordings. Clin Neurophysiol 118(1):98–104
Shanechi MM, Orsborn AL, Carmena JM (2016) Robust brainmachine interface design using optimal feedback control modeling and adaptive point process filtering. PLoS Comput Biol 12(4):e1004730
Son J, Ku J (2019) Development of brain computer interface based action observation program with functional electrical stimulation device (fes). In: The 7th international winter conference on brain-computer Interface (BCI). IEEE, Gangwon, pp 1–2
Srivastava N, Hinton G, Krizhevsky A et al (2017) Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res 15(1):1929–1958
Stevens C, Paulsen D, Yasen A, Neville H (2015) Atypical auditory refractory periods in children from lower socio-economic status backgrounds: Erp evidence for a role of selective attention. Int J Psychophysiol 95(2):156–166
Stock AK, Ness V, Beste C (2015) Complex sensorimotor transformation processes required for response selection are facilitated by the striatum. Neuroimage 123:33–41
Tabar YR, Halici U (2016) A novel deep learning approach for classification of eeg motor imagery signals. Journal of Neural Engineering 14(1):016003
Tangermann M, Müller K-R, Aertsen A, Birbaumer N, Braun C, Brunner C, Leeb R, Mehring C, Miller KJ, Müller-Putz GR, Nolte G, Pfurtscheller G, Preissl H, Schalk G, Schlögl A, Vidaurre C, Waldert S, Blankertz B (2012) Review of the bci competition iv. Front Neurosci 6:55
Te H, Yan-Fu L, Min Q (2021) A hybrid generalization network for intelligent fault diagnosis of rotating machinery under unseen working conditions. IEEE Trans Instrum Meas 70:3520011
Trottier L, Gigu P, Chaib-draa B et al (2017) Parametric exponential linear unit for deep convolutional neural networks. In: The 16th IEEE international conference on machine learning and applications (ICMLA). IEEE, Cancun, pp 207–214
Tuma T, Pantazi A, Le Gallo M et al (2016) Stochastic phase-change neurons. Nature Nanotechnology 11(8):693
Varnum ME, Blais C, Brewer GA (2016) Brewer.Social class affects musuppression during action observation. Soc Neurosci 11(4):449–454
Wu T, Hou Y, Hallett M, Zhang J, Chan P (2015) Lateralization of brain activity pattern during unilateral movement in parkinson’s disease. Hum Brain Mapp 36(5):1878–1891
Xiaochen Z, He Z, Zhongliang Z et al (2021) Performance degradation analysis and fault prognostics of solid oxide fuel cells using the data-driven method. Int J Hydrog Energy 46:18511–18523
Acknowledgements
This work was funded by the National Natural Science Foundation of China under Grant (No. 62106049, No. 61673322). The funding body played the role in supporting the experiments. The author wants to thank the members of the digital Fujian internet-of-thing laboratory of environmental monitoring in Fujian Normal University, and the brain-like robotic research group of Xiamen University for their proofreading comments. The author is very grateful to the anonymous reviewers for their constructive comments which have helped significantly in revising this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors disclosed no relevant relationships.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, Tj., Zhou, C. Lateralized modulation brought by discrepancy speed ratios of left and right arm movements during human action observation: an EEG study . Multimed Tools Appl 81, 17567–17594 (2022). https://doi.org/10.1007/s11042-022-11971-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11042-022-11971-8