Abstract
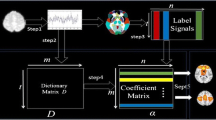
Recently, sparse representation has received a great deal of attention in voxel selection and decoding analysis of functional magnetic resonance imaging (fMRI) data. Due to contamination of large noise in fMRI data, the conventional sparse representation methods may not get stable results. Moreover, the selected activated brain regions may lose clustering effects and are less biologically interpretable. In order to overcome the above mentioned problems, we exploit the error-tolerant formulation of sparse representation and introduce multi-dimensional derivative constraints (smoothness constraints) in optimization. Two new methods are proposed in this paper. One is robust voxel selection with multi-dimensional constraint (RVSMDC). With the error-tolerant formulation and smoothness constraints on regression coefficients, RVSMDC is robust against noise/error and achieves clustering effects. To directly decode neural activities from fMRI data, we also proposed robust sparse decoding with multi-dimensional constraints (RSDMDC), which minimize the regression error of fMRI data to the task function with sparsity and smoothness constraints on regression coefficients. Numerical results validate the effectiveness of the two proposed methods.








Similar content being viewed by others
References
Bader, B. W., & Kolda, T. G. (2007). Efficient MATLAB computations with sparse and factored tensors. SIAM Journal on Scientific Computing, 30(1), 205–231.
Bandettini, P. A., Wong, E. C., Hinks, R. S., Tikofsky, R. S., & Hyde, J. S. (1992). Time course EPI of human brain function during task activation. Magnetic Resonance in Medicine, 25(2), 390–397.
Birn, R. M., Saad, Z. S., & Bandettini, P. A. (2001). Spatial heterogeneity of the nonlinear dynamics in the fMRI BOLD response. NeuroImage, 14(4), 817–826.
Bunea, F., She, Y., Ombao, H., Gongvatana, A., Devlin, K., & Cohen, R. (2011). Penalized least squares regression methods and applications to neuroimaging. NeuroImage, 55(4), 1519–1527.
Buxton, R. B., Wong, E. C., & Frank, L. R. (1998). Dynamics of blood flow and oxygenation changes during brain activation: The balloon model. Magnetic Resonance in Medicine, 39(6), 855–864.
Carlson, T. A., Schrater, P., & He, S. (2003). Patterns of activity in the categorical representations of objects. Journal of Cognitive Neuroscience, 15(5), 704–717.
Carroll, M. K., Cecchi, G. A., Rish, I., Garg, R., & Rao, A. R. (2009). Prediction and interpretation of distributed neural activity with sparse models. NeuroImage, 44(1), 112–122.
Cohen, M. S., & Bookheimer, S. Y. (1994). Localization of brain function using magnetic resonance imaging. Trends in Neurosciences, 17(7), 268–277.
Cox, D. D., & Savoy, R. L. (2003). Functional magnetic resonance imaging (fMRI) brain reading: Detecting and classifying distributed patterns of fMRI activity in human visual cortex. NeuroImage, 19(2), 261–270.
Friston, K. J., Mechelli, A., Turner, R., & Price, C. J. (2000). Nonlinear responses in fMRI: The balloon model, volterra kernels, and other hemodynamics. NeuroImage, 12(4), 466–477.
Friston, K. J., Ashburner, J. T., Kiebel, S. J., Nichols, T. E., & Penny, W. D. (2007). Statistical parametric mapping: The analysis of funtional brain images. Amsterdam: Academic Press.
Grosenick, L., Greer, S., & Knutson, B. (2008). Interpretable classifiers for fMRI improve prediction of purchases. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 16(6), 539–548.
Guo, X., Wan, Q., Chang, C., & Lam, E. Y. (2010). Source localization using a sparse representation framework to achieve superresolution. Multidimensional Systems and Signal Processing, 21(4), 391–402.
Harrison, L. M., Penny, W., Daunizeau, J., & Friston, K. J. (2008). Diffusion-based spatial priors for functional magnetic resonance images. NeuroImage, 41(2–3), 408.
Jiang, L., & Yin, H. (2012). Bregman iteration algorithm for sparse nonnegative matrix factorizations via alternating l1-norm minimization. Multidimensional Systems and Signal Processing, 23(3), 315–328.
LaConte, S., Strother, S., Cherkassky, V., Anderson, J., & Hu, X. (2005). Support vector machines for temporal classification of block design fMRI data. NeuroImage, 26(2), 317.
Li, Y., Namburi, P., Yu, Z., Guan, C., Feng, J., & Gu, Z. (2009). Voxel selection in fMRI data analysis based on sparse representation. IEEE Transactions on Biomedical Engineering, 56(10), 2439–2451.
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T., & Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412(6843), 150–157.
Logothetis, N. K. (2003). The underpinnings of the BOLD functional magnetic resonance imaging signal. The Journal of Neuroscience, 23(10), 3963–3971.
Mitchell, T. M., Hutchinson, R., Niculescu, R. S., Pereira, F., Wang, X., Just, M., et al. (2004). Learning to decode cognitive states from brain images. Machine Learning, 57(1), 145–175.
Oikonomou, V. P., Blekas, K., & Astrakas, L. (2012). A sparse and spatially constrained generative regression model for fMRI data analysis. IEEE Transactions on Biomedical Engineering, 59(1), 58–67.
O’toole, A. J., Jiang, F., Abdi, H., & Haxby, J. V. (2005). Partially distributed representations of objects and faces in ventral temporal cortex. Journal of Cognitive Neuroscience, 17(4), 580–590.
Pizzagalli, D. A., Sherwood, R. J., Henriques, J. B., & Davidson, R. J. (2005). Frontal brain asymmetry and reward responsiveness a source-localization study. Psychological Science, 16(10), 805–813.
Rorden, C., & Karnath, H. O. (2004). Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nature Reviews Neuroscience, 5(10), 812–819.
Spiridon, M., & Kanwisher, N. (2002). How distributed is visual category information in human occipito-temporal cortex? An fMRI study. Neuron, 35(6), 1157–1166.
Stephan, K. E., Kasper, L., Harrison, L. M., Daunizeau, J., den Ouden, H. E. M., Breakspear, M., et al. (2008). Nonlinear dynamic causal models for fMRI. NeuroImage, 42(2), 649–662.
Strother, S. C., Anderson, J., Hansen, L. K., Kjems, U., Kustra, R., Sidtis, J., et al. (2002). The quantitative evaluation of functional neuroimaging experiments: The NPAIRS data analysis framework. NeuroImage, 15(4), 747–771.
Tibshirani, R. (1996). Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society, Series B: Methodological, 58(1), 267–288.
Vazquez, A. L., & Noll, D. C. (1998). Nonlinear aspects of the BOLD response in functional MRI. NeuroImage, 7(2), 108–118.
Yamashita, O., Sato, M., Yoshioka, T., Tong, F., & Kamitani, Y. (2008). Sparse estimation automatically selects voxels relevant for the decoding of fMRI activity patterns. NeuroImage, 42(4), 1414–1429.
Yetkin, F. Z., McAuliffe, T. L., Cox, R., & Haughton, V. M. (1996). Test-retest precision of functional MR in sensory and motor task activation. American Journal of Neuroradiology, 17(1), 95–98.
Yu, Z., Gu, Z., & Li, Y. (2011). A sparse voxel selection approach for fMRI data analysis with multi-dimensional derivative constraints. In Multidimensional (nD) systems (nDs), (2011). 7th international workshop on. IEEE, 2011, pp. 1–4.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China under Grants 61105121, 61175114, 91120305, the Natural Science Foundation of Guangdong under Grants S2012020010945, the Fundamental Research Funds for the Central Universities, SCUT under Grants 2012ZG0008, 2013ZZ0040, the National High-tech R&D Program of China (863 Program) under Grant 2012AA011601, and High Level Talent Project of Guangdong Province, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Z., Feng, B., Gu, Z. et al. Voxel selection and neural decoding of fMRI data based on robust sparse programming with multi-dimensional derivative constraints. Multidim Syst Sign Process 26, 225–241 (2015). https://doi.org/10.1007/s11045-013-0254-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11045-013-0254-3