Abstract
Stimulation of the vagus nerve potentially decreases the risk of sudden cardiac death. An improvement of the technique would be its regulation using the heart rate (HR) as a feedback variable. We address the possibility of closed-loop control of the HR, focusing on the stimulation parameters, nerve fibre populations and the reproducibility of the cardiovascular response. The response to electrical stimulation of the vagus nerve was studied in seven acute experiments on pigs. Feedback regulation of the HR over periods of 5 min was carried out. Three main populations of myelinated fibres were found. The performance of the controller was significantly better at amplitudes higher than those needed for stimulation of the myelinated components only. A 18% change in the duration of the RR interval could be controlled in all experiments. The possibility of closed-loop control of the HR seems to be promising.






Similar content being viewed by others
References
Agostoni E, Chinnock JE, Daly MDB, Murray JG (1957) Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol (Lond) 135:182–205
Ali II, Pirzada NA, Kanjwal Y, Wannamaker B, Medhkour A, Koltz MT, Vaughn BV (2004) Complete heart block with ventricular asystole during left vagus nerve stimulation for epilepsy. Epilepsy Behav 5:768–771
Ben Menachem E (2002) Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol 1:477–482
Bilgutay AM, Bilgutay IM, Merkel FK, Lillehei CW (1968) Vagal tuning—a new concept in treatment of supraventricular arrhythmias angina pectoris and heart failure. J Thoracic Cardiovasc Surg 56:71–82
Billman GE, Hoskins RS, Randall DC, Randall WC, Hamlin RL, Lin YC (1989) Selective vagal postganglionic innervation of the sinoatrial and atrioventricular nodes in the non-human primate. J Auton Nerv Syst 26:27–36
Bishop O (2000) Understand electronic control systems. Newnes, Oxford, UK
Buckley NM, Gootman PM, Brazeau P, Matanic BP, Frasier ID, Gentles EL (1979) Cardiovascular function in anesthetized miniature swine. Lab Anim Sci 29(2):200–208
Cheng ZX, Powley TL (2000) Nucleus ambiguus projections to cardiac ganglia of rat atria: An anterograde tracing study. J Comp Neurol 424:588–606
Cohn AE, Lewis T (1913) The predominant influence of the left vagus nerve upon conduction between the auricles and ventricles in the dog. J Exp Med 18:739–747
Dexter F, Levy MN, Rudy Y (1989) Mathematical-model of the changes in heart-rate elicited by vagal-stimulation. Circ Res 65:1330–1339
Erlanger J, Gasser HS (1937) Electrical signs of nervous activity. University of Pennsylvania Press, Philadelphia, PA
Evans MS, Verma-Ahuja S, Naritoku DK, Espinosa JA (2004) Intraoperative human vagus nerve compound action potentials. Acta Neurol Scand 110:232–238
Ford TW, Mcwilliam PN (1986) The effects of electrical-stimulation of myelinated and nonmyelinated vagal fibers on heart-rate in the rabbit. J Physiol (Lond) 380:341–347
Frei MG, Osorio I (2001) Left vagus nerve stimulation with the neurocybernetic prosthesis has complex effects on heart rate and on its variability in humans. Epilepsia 42:1007–1016
Furlan R, Diedrich A, Rimoldi A, Palazzolo L, Porta C, Diedrich L, Harris PA, Sleight P, Biagioni I, Robertson D, Bernardi L (2003) Effects of unilateral and bilateral carotid baroreflex stimulation on cardiac and neural sympathetic discharge oscillatory patterns. Circulation 108(6):717–23
Haugland M. (1996) A flexible method for fabrication of nerve cuff electrodes. In: Proceedings of the 18th annual international conference of the IEEE, Bridging disciplines for biomedicine. Eng Med Biol Soc 1:359–360
Jones JFX, Wang Y, Jordan D (1995) Heart-rate responses to selective stimulation of cardiac vagal-C fibers in anesthetized cats, rats and rabbits. J Physiol (Lond) 489:203–214
Jones JFX, Wang Y, Jordan D (1998) Activity of C fibre cardiac vagal efferents in anaesthetized cats and rats. J Physiol (Lond) 507:869–880
Kawada T, Ikeda Y, Sugimachi M, Shishido T, Kawaguchi O, Yamazaki T, Alexander J, Sunagawa K (1996) Bidirectional augmentation of heart rate regulation by autonomic nervous system in rabbits. Am J Physiol Heart Circ Physiol 40:H288–H295
Koo B, Ham SD, Sood S, Tarver B (2001) Human vagus nerve electrophysiology—a guide to vagus nerve stimulation parameters. J Clin Neurophysiol 18:429–433
Kunze DL (1972) Reflex discharge patterns of cardiac vagal efferent fibers. J Physiol (Lond) 222:1–15
Kuo BC (1991) Automatic control systems. Prentice Hall, Englewood Cliffs, NJ
Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K (2004) Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109:120–124
Mancia G, Ferrari A, Gregorini L, Valentini R, Ludbrook J, Zanchetti A (1977) Circulatory reflexes from carotid and extracarotid baroreceptor areas in man. Circ Results 41(3):309–15
Matheny RG, Shaar CJ (1997) Vagus nerve stimulation as a method to temporarily slow or arrest the heart. Ann Thorac Surg 63:S28–S29
Middleton S, Middleton HH, Grundfest H (1950) Spike potentials and cardiac effects of mammalian vagus nerve. Am J Physiol 162:545–552
Parker P, Celler BG, Potter EK, Mccloskey DI (1984) Vagal-stimulation and cardiac slowing. J Auton Nerv Syst 11:226–231
Piterman L, Zimmet H, Krum H, Tonkin A, Yallop J (2005) Chronic heart failure—optimising care in general practice. Aust Fam Physician 34(7):547–553
Prakash P, Safanie AH (1967) Asymmetrical distribution of aortic nerve fibers in pig. Anat Rec 158:51–57
Randall WC, Milosavljevic M, Wurster RD, Geis GS, Ardell JL (1986) Selective vagal innervation of the heart. Ann Clin Lab Sci 16:198–208
Routledge HC, Chowdhary S, Townend JN (2002) Heart rate variability—a therapeutic target? J Clin Pharm Ther 27(2):85–92
Sloan TB (1998) Anesthetic effects on electrophysiologic recordings. J Clin Neurophysiol 15(3):217–226
Swindle MM (1998) Surgery, anaesthesia, and experimental techniques in Swine. Iowa State University Press, Ames, IA
Tosato M, Yoshida K, Toft E, Struijk JJ (2005) Characterization of the cardiac response to vagal nerve stimulation. In: 2nd international IEEE EMBS conference on March 16–19, Neural Eng 1:540–542
Vanoli E, Deferrari GM, Strambabadiale M, Hull SS, Foreman RD, Schwartz PJ (1991) Vagal-stimulation and prevention of sudden-death in conscious dogs with a healed myocardial-infarction. Circ Res 68:1471–1481
Waninger MS, Bourland JD, Geddes LA, Schoenlein WE, Graber G, Weirich WE, Wodicka GR (2000) Electrophysiological control of ventricular rate during atrial fibrillation. Pace-Pacing Clin Electrophysiol 23:1239–1244
Warner HR, Cox A (1962) A mathematical model of heart rate control by sympathetic and vagus efferent information. J Appl Physiol 17:349–355
Zhang YH, Mowrey KA, Zhuang SW, Wallick DW, Popovic ZB, Mazgalev TN (2002) Optimal ventricular rate slowing during atrial fibrillation by feedback AV nodal-selective vagal stimulation. Am J Physiol Heart Circ Physiol 282:H1102–H1110
Acknowledgements
The authors thank Dr Henrik Barlebo for access to the Biolab and helping to make this study possible. We also thank Ole Sørensen, Torben Madsen and Jens Sørensen, for their useful technical suggestions and help in handling the animals. This work was supported by the European Commission for the NeuralPRO Network (FP5-Program, Research and Training Network).
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support: European Commission for the NeuralPRO (FP5-Program, Research and Training Network)
Appendix: VNS-control algorithm
Appendix: VNS-control algorithm
Control theory is a vast subject and it is extensively covered in most engineering educations and by many books (see e.g. [6, 22]).
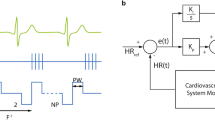
A system is closed-loop controlled when at least one of its inputs depend on its output(s). Otherwise, the system is said to be in open loop. We chose to close the loop in the stimulator-vagus-heart system by continuously measuring the variable we wanted to control—the RR interval—and by feeding it back to the stimulator through a controller (Fig. 1). At each heart beat, the controller recalculates the difference between measured and target value (tracking error) and updates the input to the stimulator (the stimulation frequency) accordingly.
The proportional-integral-derivative algorithm (PID) is often a useful choice when the system to be controlled is too difficult to model, which makes the design of an optimal ad hoc controller impossible. The output of the controller is the sum of three actions (proportional, integrative and derivative), the values of which are based on the behaviour of the tracking error. When the derivative action is not used, it becomes a PI.
The proportional action deals with sudden changes in the system output. It is proportional to the tracking error.
The integrative action tries to reduce any steady tracking error. It is based on the integral of the tracking error, which is continuously computed. At any given instant, the value of this action will be proportional to the current value of the integral.
The derivative action attempts to dampen oscillations of the system output. At any given instant, the derivative is estimated by the difference between the current and the previous value of the feedback variable. Since rapid variations result in big differences between contiguous values, they will result in a strong derivative action, aimed at bringing the tracking error back to zero.
The Bang–bang or on-off strategy consists in turning the stimulation on when the tracking error is positive and turning it off when it is zero or within an accepted range.
Rights and permissions
About this article
Cite this article
Tosato, M., Yoshida, K., Toft, E. et al. Closed-loop control of the heart rate by electrical stimulation of the vagus nerve. Med Bio Eng Comput 44, 161–169 (2006). https://doi.org/10.1007/s11517-006-0037-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-006-0037-1