Abstract
We have expanded a former compartmental model of blood glucose regulation for healthy and type 2 diabetic subjects. The former model was a detailed physiological model which considered the interactions of three substances, glucose, insulin and glucagon on regulating the blood sugar. The main drawback of the former model was its restriction on the route of glucose entrance to the body which was limited to the intravenous glucose injection. To handle the oral glucose intake, we have added a model of glucose absorption in the gastrointestinal tract to the former model to address the resultant variations of blood glucose concentrations following an oral glucose intake. Another model representing the incretins production in the gastrointestinal tract along with their hormonal effects on boosting pancreatic insulin production is also added to the former model. We have used two sets of clinical data obtained during oral glucose tolerance test and isoglycemic intravenous glucose infusion test from both type 2 diabetic and healthy subjects to estimate the model parameters and to validate the model results. The estimation of model parameters is accomplished through solving a nonlinear optimization problem. The results show acceptable precision of the estimated model parameters and demonstrate the capability of the model in accurate prediction of the body response during the clinical studies.







Similar content being viewed by others
References
Ackerman E, Gatewood LC, Rosevear JW, Molnar GD (1965) Model studies of blood-glucose regulation. Bull Math Biophys 27(Suppl):21–37
Ajmera I, Swat M, Laibe C, Novere NL, Chelliah V (2013) The impact of mathematical modeling on the understanding of diabetes and related complications. CPT Pharmacometrics Syst Pharmacol 2:e54. doi:10.1038/psp.2013.30
Alvehag K (2006) Glucose regulation. A Mathematical Model. KTH—Royal Institute of Technology, Stockholm
Alzaid AA, Dinneen SF, Turk DJ, Caumo A, Cobelli C, Rizza RA (1994) Assessment of insulin action and glucose effectiveness in diabetic and nondiabetic humans. J Clin Investig 94:2341–2348. doi:10.1172/JCI117599
Basu A, Alzaid A, Dinneen S, Caumo A, Cobelli C, Rizza RA (1996) Effects of a change in the pattern of insulin delivery on carbohydrate tolerance in diabetic and nondiabetic humans in the presence of differing degrees of insulin resistance. J Clin Investig 97:2351–2361. doi:10.1172/JCI118678
Basu A, Dalla Man C, Basu R, Toffolo G, Cobelli C, Rizza RA (2009) Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care 32:866–872. doi:10.2337/dc08-1826
Basu R, Chandramouli V, Dicke B, Landau B, Rizza R (2005) Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 54:1942–1948
Basu R, Schwenk WF, Rizza RA (2004) Both fasting glucose production and disappearance are abnormal in people with mild and severe type 2 diabetes. Am J Physiol Endocrinol Metab 287:E55–E62. doi:10.1152/ajpendo.00549.2003
Bergman RN, Phillips LS, Cobelli C (1981) Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Investig 68:1456–1467
Bolie VW (1961) Coefficients of normal blood glucose regulation. J Appl Physiol 16:783–788
Borra R, Lautamaki R, Parkkola R, Komu M, Sijens PE, Hallsten K, Bergman J, Iozzo P, Nuutila P (2008) Inverse association between liver fat content and hepatic glucose uptake in patients with type 2 diabetes mellitus. Metab Clin Exp 57:1445–1451. doi:10.1016/j.metabol.2008.05.015
Cedersund G, Stralfors P (2009) Putting the pieces together in diabetes research: towards a hierarchical model of whole-body glucose homeostasis. Eur J Pharm Sci 36:91–104. doi:10.1016/j.ejps.2008.10.027ER
Cobelli C, Mari A (1983) Validation of mathematical models of complex endocrine-metabolic systems. A case study on a model of glucose regulation. Med Biol Eng Comput 21:390–399
Costanzo LS (2002) Physiology. vol Book, Whole. Saunders, Philadelphia
Creutzfeldt W (1979) The incretin concept today. Diabetologia 16:75–85
Dalla Man C, Camilleri M, Cobelli C (2006) A system model of oral glucose absorption: validation on gold standard data. IEEE Trans Biomed Eng 53:2472–2478. doi:10.1109/TBME.2006.883792
Dalla Man C, Caumo A, Cobelli C (2002) The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 49:419–429. doi:10.1109/10.995680
Dalla Man C, Rizza RA, Cobelli C (2007) Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng 54:1740–1749. doi:10.1109/TBME.2007.893506
Dalla Man C, Yarasheski KE, Caumo A, Robertson H, Toffolo G, Polonsky KS, Cobelli C (2005) Insulin sensitivity by oral glucose minimal models: validation against clamp. Am J Physiol Endocrinol Metab 289:E954–E959. doi:10.1152/ajpendo.00076.2005
DeFronzo RA (2004) Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 88:787–835. doi:10.1016/j.mcna.2004.04.013
DelPrato S, Matsuda M, Simonson DC, Groop LC, Sheehan P, Leonetti F, Bonadonna RC, DeFronzo RA (1997) Studies on the mass action effect of glucose in NIDDM and IDDM: evidence for glucose resistance. Diabetologia 40:687–697
Dua P, Doyle F III, Pistikopoulos E (2009) Multi-objective blood glucose control for type 1 diabetes. Med Biol Eng Comput 47:343–352. doi:10.1007/s11517-009-0453-0
Elrick H, Stimmler L, Hlad CJ, Arai Y (1964) Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 24:1076–1082. doi:10.1210/jcem-24-10-1076
Fabietti P, Canonico V, Federici M, Benedetti M, Sarti E (2006) Control oriented model of insulin and glucose dynamics in type 1 diabetics. Med Biol Eng Comput 44:69–78. doi:10.1007/s11517-005-0012-2
Fery F (1994) Role of hepatic glucose production and glucose uptake in the pathogenesis of fasting hyperglycemia in type 2 diabetes: normalization of glucose kinetics by short-term fasting. J Clin Endocrinol Metab 78:536–542
Groop LC, Widen E, Ferrannini E (1993) Insulin resistance and insulin deficiency in the pathogenesis of type 2 (non-insulin-dependent) diabetes mellitus: errors of metabolism or of methods? Diabetologia 36:1326–1331
Guillausseau PJ, Meas T, Virally M, Laloi-Michelin M, Medeau V, Kevorkian JP (2008) Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes Metab 34(Suppl 2):S43–S48. doi:10.1016/S1262-3636(08)73394-9
Guyton JR, Foster RO, Soeldner JS, Tan MH, Kahn CB, Koncz L, Gleason RE (1978) Model of glucose-insulin homeostasis in man that incorporates heterogeneous fast pool theory of pancreatic insulin release. Diabetes 27:1027–1042
Hernandez-Ordonez M, Campos-Delgado DU (2008) An extension to the compartmental model of type 1 diabetic patients to reproduce exercise periods with glycogen depletion and replenishment. J Biomech 41:744–752. doi:10.1016/j.jbiomech.2007.11.028
Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Orsini Federici M, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME (2004) Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas 25:905–920
Iozzo P, Hallsten K, Oikonen V, Virtanen KA, Kemppainen J, Solin O, Ferrannini E, Knuuti J, Nuutila P (2003) Insulin-mediated hepatic glucose uptake is impaired in type 2 diabetes: evidence for a relationship with glycemic control. J Clin Endocrinol Metab 88:2055–2060
Kieffer TJ, Francis Habener J (1999) The glucagon-like peptides. Endocr Rev 20:876–913. doi:10.1210/er.20.6.876
Kipnis MJ, Perley DM (1967) Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects*. J Clin Investig 46:1954–1962. doi:10.1172/JCI105685
Knop FK, Vilsboll T, Hojberg PV, Larsen S, Madsbad S, Volund A, Holst JJ, Krarup T (2007) Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 56:1951–1959. doi:10.2337/db07-0100
Landahl HD, Grodsky GM (1982) Comparison of models of insulin release. Bull Math Biol 44:399–409
Leahy JL (2005) Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36:197–209. doi:10.1016/j.arcmed.2005.01.003
Makroglou A, Li J, Kuang Y (2006) Mathematical models and software tools for the glucose-insulin regulatory system and diabetes: an overview. Selected Papers, The Third International Conference on the Numerical Solutions of Volterra and Delay Equations 56:559–573. doi:10.1016/j.apnum.2005.04.023
Marchetti G, Barolo M, Jovanovic L, Zisser H, Seborg DE (2008) An improved PID switching control strategy for type 1 diabetes. IEEE Trans Biomed Eng 55:857–865. doi:10.1109/TBME.2008.915665
Mari A (2002) Mathematical modeling in glucose metabolism and insulin secretion. Curr Opin Clin Nutr Metab Care 5:495–501
McIntyre N, Holdsworth CD, Turner DS (1965) Intestinal factors in the control of insulin secretion. J Clin Endocrinol Metab 25:1317–1324. doi:10.1210/jcem-25-10-1317
Nielsen MF, Basu R, Wise S, Caumo A, Cobelli C, Rizza RA (1998) Normal glucose-induced suppression of glucose production but impaired stimulation of glucose disposal in type 2 diabetes: evidence for a concentration-dependent defect in uptake. Diabetes 47:1735–1747
Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, Frank BH, Galloway JA, Van Cauter E (1988) Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med 318:1231–1239. doi:10.1056/NEJM198805123181903
Ramprasad Y, Rangaiah GP, Lakshminarayanan S (2006) Enhanced IMC for glucose control in type I diabetics using a detailed physiological model. Food Bioprod Process 84:227–236. doi:10.1205/fbp.05070ER
Seino Y (2011) Understanding the incretin effect. J Clin Endocrinol Metab 96:934–935. doi:10.1210/jc.2011-0329
Seino Y, Fukushima M, Yabe D (2010) GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Investig 1:8–23. doi:10.1111/j.2040-1124.2010.00022.x
Shapiro ET, Tillil H, Miller MA, Frank BH, Galloway JA, Rubenstein AH, Polonsky KS (1987) Insulin secretion and clearance. Comparison after oral and intravenous glucose. Diabetes 36:1365–1371. doi:10.2337/diabetes.36.12.1365
Sorensen JT (1985) A physiological model of glucose metabolism in man and its use to design and assess improved insulin therapies for diabetes. Massachusetts Institute of Technology, Cambridge
Thomaseth K, Pavan A, Berria R, Glass L, DeFronzo R, Gastaldelli A (2008) Model-based assessment of insulin sensitivity of glucose disposal and endogenous glucose production from double-tracer oral glucose tolerance test. Comput Methods Programs Biomed 89:132–140. doi:10.1016/j.cmpb.2007.06.003
Vahidi O, Kwok KE, Gopaluni RB, Sun L (2011) Developing a physiological model for type 2 diabetes mellitus. Biochem Eng J 55:7–16. doi:10.1016/j.bej.2011.02.019
Weyer C, Bogardus C, Mott DM, Pratley RE (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Investig 104:787–794. doi:10.1172/JCI7231
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The following nomenclature is adopted throughout the Sorensen model description.
Model variables in the glucose sub-model
- D :
-
Oral glucose amount (mg)
- G :
-
Glucose concentration (mg/dl)
- M :
-
Multiplier of metabolic rates (dimensionless)
- q :
-
Glucose amount in GI tract (mg)
- Q :
-
Vascular blood flow rate (dl/min)
- r :
-
Metabolic production or consumption rate (mg/min)
- Ra :
-
Rate of glucose appearance in the blood stream (mg/min)
- T :
-
Transcapillary diffusion time constant (min)
- t :
-
Time (min)
- V :
-
Volume (dl)
Model variables in the insulin sub-model
- I :
-
Insulin concentration (mU/l)
- M :
-
Multiplier of metabolic rates (dimensionless)
- m :
-
Labile insulin mass (U)
- P :
-
Potentiator (dimensionless)
- Q :
-
Vascular blood flow rate (l/min)
- R :
-
Inhibitor (dimensionless)
- r :
-
Metabolic production or consumption rate (mU/min)
- S :
-
Insulin secretion rate (U/min)
- T :
-
Transcapillary diffusion time constant (min)
- t :
-
Time (min)
- V :
-
Volume (l)
- X :
-
Glucose-enhanced excitation factor (dimensionless)
- Y :
-
Intermediate variable (dimensionless)
Model variables in the glucagon sub-model
- \(\varGamma\) :
-
Normalized glucagon concentration (dimensionless)
- M :
-
Multiplier of metabolic rates (dimensionless)
- r :
-
Metabolic production or consumption rate (dl/min)
- V :
-
Volume (dl)
- t :
-
Time (min)
Model variables in the incretin sub-model
- \(\varPsi\) :
-
Incretin concentration (pmol/l)
- r :
-
Metabolic production or consumption rate (pmol/min)
- V :
-
Volume (l)
- t :
-
Time (min)
First superscript
- Γ:
-
Glucagon
- Ψ:
-
Incretins
- B :
-
Basal condition
- G :
-
Glucose
- I :
-
Insulin
Second superscript
- \(\infty\) :
-
Final steady-state value
Metabolic rate subscripts
- BGU:
-
Brain glucose uptake
- GGU:
-
Gut glucose uptake
- HGP:
-
Hepatic glucose production
- HGU:
-
Hepatic glucose uptake
- \(I\varPsi R\) :
-
Intestinal incretins release
- IVG:
-
Intravenous glucose infusion
- IVI:
-
Intravenous insulin infusion
- KGE:
-
Kidney glucose excretion
- KIC:
-
Kidney insulin clearance
- LIC:
-
Liver insulin clearance
- \(M\varGamma C\) :
-
Metabolic glucagon clearance
- \(P\varGamma C\) :
-
Plasma glucagon clearance
- \(P\varPsi C\) :
-
Plasma incretins clearance
- \(P\varGamma R\) :
-
Pancreatic glucagon release
- PGU:
-
Peripheral glucose uptake
- PIC:
-
Peripheral insulin clearance
- PIR:
-
Pancreatic insulin release
- RBCU:
-
Red blood cell glucose uptake
First subscripts
- A :
-
Hepatic artery
- B :
-
Brain
- G :
-
Gut
- H :
-
Heart and lungs
- L :
-
Liver
- P :
-
Periphery
- S :
-
Stomach
- \(\infty\) :
-
Final steady-state value
Second subscripts (if required)
- C :
-
Capillary space
- F :
-
Interstitial fluid space
- l :
-
Liquid
- s :
-
Solid
1.1 Glucose sub-model
The mass balance equation over each compartment in the glucose sub-model results in following equations:
The metabolic rates for the glucose sub-model are summarized below:
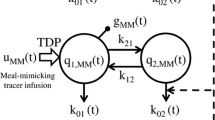
The model of glucose absorption in the GI tract proposed by Dalla Man et al. [16] is added to the glucose sub-model. The model equations are:
where \(\delta \left( t \right)\) is the impulse function.
1.2 Insulin sub-model
The mass balance equation over the compartments in the insulin sub-model results in following equations:
The metabolic rates for the insulin sub-model are summarized below:
As mentioned, the pancreatic insulin release model used in the Sorensen model has been proposed by Landahl and Grodsky [35]. The aim of Landahl and Grodsky’s model is to mimic the biphasic behavior of pancreatic insulin secretion in response to a glucose stimulus. In this model, a small labile insulin compartment is assumed to exchange insulin with a large storage compartment. The rate at which insulin flows into the labile compartment is regulated by a glucose-stimulated factor, P. The rate of insulin secretion, S, is dependent on glucose concentration, the amount of labile insulin, m, and the difference between the instantaneous level of glucose-enhanced excitation factor, X, and its inhibitor, R. This functionality provides a mathematical description of the pancreas biphasic response to a glucose stimulus. The first-phase insulin release is caused by an instantaneous increase in the glucose-enhanced excitation factor (X) followed by a rapid increase in its inhibitor (R). The second-phase release results from the direct dependence of the insulin secretion rate (S) on the glucose stimulus and the gradual increase in the level of the labile compartment filling factor (P).
The mass balance equation over each compartment results in:
It is assumed that the capacity of the storage compartment is large enough and remains at steady state. For a glucose concentration of zero, P is set to zero. Therefore, the steady-state mass balance equation around the storage compartment is:
where \(m_{0}\) is the labile insulin quantity at a glucose concentration of zero. The rest of the equations for the pancreas model are:
\(P_{\infty }\) and Y reflect the glucose-induced stimulation effects on the liable compartment filling factor and the insulin secretion rate, respectively.
1.3 Glucagon sub-model
The glucagon sub-model has one mass balance equation over the whole body as follows:
The metabolic rates for the glucagon sub-model are summarized below:
1.4 Incretin sub-model
Similar to the glucagon sub-model, the incretin sub-model has one compartment. The incretin model equations comprise two ordinary differential equations, one represents the production of the incretins following the presence of the glucose in the small intestine and the other one represents the mass balance over the compartment. The incretin production is calculated from the following differential equation:
where \(\psi\) is the amount of produced incretins, \(k_{\text{empt}} q_{Sl}\) is the rate of glucose entrance to the small intestine, \(r_{I\varPsi P}\) is the rate of incretin absorption into the blood stream, and \(\varsigma\) is a constant.
\(r_{I\varPsi P}\) is calculated from the following equation:
where \(\tau_{\varPsi }\) is the time constant of the incretin absorption process into the blood stream. The mass balance equation over the incretin compartment results in:
where \(V^{\varPsi }\) is the incretin distribution volume, \(\varPsi\) is the blood incretin concentration, and \(r_{P\varPsi C}\) is the rate of plasma incretin clearance which depends on the incretin concentration. The clearance rate is calculated from the following equation:
where \(r_{M\varPsi C}\) is the mean incretin clearance rate and is a constant.
The model constant parameters are available in [49].
Rights and permissions
About this article
Cite this article
Vahidi, O., Kwok, K.E., Gopaluni, R.B. et al. A comprehensive compartmental model of blood glucose regulation for healthy and type 2 diabetic subjects. Med Biol Eng Comput 54, 1383–1398 (2016). https://doi.org/10.1007/s11517-015-1406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-015-1406-4