Abstract
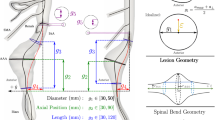
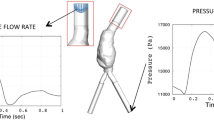
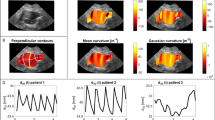
Longitudinal studies of vascular diseases often need to establish correspondence between follow-up images, as the diseased regions may change shape over time. In addition, spatial data structures should be taken into account in the statistical analyses to avoid inferential errors. This study investigates the association between hemodynamics and thrombus growth in abdominal aortic aneurysms (AAAs) while emphasizing on the abovementioned methodological issues. Six AAA surfaces and their follow-ups were three-dimensionally reconstructed from computed-tomography images. AAA surfaces were mapped onto a rectangular grid which allowed identification of corresponding regions between follow-ups. Local thrombus thickness was measured at initial and follow-up surfaces and computational fluid dynamic simulations provided time-average wall shear stress (TAWSS), oscillatory shear index (OSI), and relative residence time. Six Bayesian regression models, which account for spatially correlated measurements, were employed to explore associations between hemodynamics and thrombus growth. Results suggest that spatial regression models based on TAWSS and OSI offer superior predictive performance for thrombus growth relative to alternative specifications. Ignoring the spatial data structure may lead to improper assessment with regard to predictor significance.










Similar content being viewed by others
References
Adamson RH (1993) Microvascular endothelial cell shape and size in situ. Microvasc Res 46:77–88. doi:10.1006/mvre.1993.1036
Antiga L, Steinman DA (2004) Robust and objective decomposition and mapping of bifurcating vessels. IEEE Trans Med Imaging 23:704–713
Arzani A, Shadden SC (2016) Characterizations and correlations of wall shear stress in aneurysmal flow. J Biomech Eng. doi:10.1115/1.4032056
Arzani A, Suh GY, Dalman RL, Shadden SC (2014) A longitudinal comparison of hemodynamics and intraluminal thrombus deposition in abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol 307:H1786–H1795. doi:10.1152/ajpheart.00461.2014
Baek S, Zambrano BA, Choi J, Lim C-Y (2014) Growth prediction of abdominal aortic aneurysms and its association of intraluminal thrombus. In: 11th world congress on computational mechanics, Barcelona, Spain, July 20–25, 2014
Banerjee S, Carlin BP, Gelfand AE (2014) Hierarchical modeling and analysis of spatial data, 2nd edn. Chapman & Hall/CRC Monographs on Statistics and Applied Probability
Biasetti J, Gasser TC, Auer M, Hedin U, Labruto F (2010) Hemodynamics of the normal aorta compared to fusiform and saccular abdominal aortic aneurysms with emphasis on a potential thrombus formation mechanism. Ann Biomed Eng 38:380–390. doi:10.1007/s10439-009-9843-6
Biasetti J, Hussain F, Gasser TC (2011) Blood flow and coherent vortices in the normal and aneurysmatic aortas: a fluid dynamical approach to intra-luminal thrombus formation. J R Soc Interface 8:1449–1461. doi:10.1098/rsif.2011.0041
Colantuoni G, Hellums JD, Moake JL, Alfrey CP Jr (1977) The response of human platelets to shear stress at short exposure times. Trans Am Soc Artif Intern Organs 23:626–631
De Santis G, Mortier P, De Beule M, Segers P, Verdonck P, Verhegghe B (2010) Patient-specific computational fluid dynamics: structured mesh generation from coronary angiography. Med Biol Eng Comput 48:371–380. doi:10.1007/s11517-010-0583-4
Dewey CF Jr, Bussolari SR, Gimbrone MA Jr, Davies PF (1981) The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103:177–185
Di Martino ES, Bohra A, Vande Geest JP, Gupta N, Makaroun MS, Vorp DA (2006) Biomechanical properties of ruptured versus electively repaired abdominal aortic aneurysm wall tissue. J Vasc Surg 43:570–576; discussion 576. doi:10.1016/j.jvs.2005.10.072
Doyle B, Miller K, Wittek A, Nielsen PMF (2014) From detection to rupture: a serial computational fluid dynamics case study of a rapidly expanding, patient-specific, ruptured abdominal aortic aneurysm. In: Springer (ed) Computational biomechanics for medicine, pp 53–68
Folkesson M, Silveira A, Eriksson P, Swedenborg J (2011) Protease activity in the multi-layered intra-luminal thrombus of abdominal aortic aneurysms. Atherosclerosis 218:294–299. doi:10.1016/j.atherosclerosis.2011.05.002
Fraser KH, Meagher S, Blake JR, Easson WJ, Hoskins PR (2008) Characterization of an abdominal aortic velocity waveform in patients with abdominal aortic aneurysm. Ultrasound Med Biol 34:73–80. doi:10.1016/j.ultrasmedbio.2007.06.015
Hansen KB, Arzani A, Shadden SC (2015) Mechanical platelet activation potential in abdominal aortic aneurysms. J Biomech Eng 137:041005. doi:10.1115/1.4029580
He X, Ku DN (1996) Pulsatile flow in the human left coronary artery bifurcation: average conditions. J Biomech Eng 118:74–82
Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH (2004) Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol 286:H1916–H1922. doi:10.1152/ajpheart.00897.2003
Hodges JS, Reich BJ (2010) Adding spatially-correlated errors can mess up the fixed effect you love. Am Stat 64:325–334. doi:10.1198/tast.2010.10052
Hughes J (2014) ngspatial: a package for fitting the centered autologistic and sparse spatial generalized linear mixed models for areal data. R J 6:81–95
Hughes J, Haran M (2013) Dimension reduction and alleviation of confounding for spatial generalized linear mixed models. J R Stat Soc B 75:139–159. doi:10.1111/j.1467-9868.2012.01041.x
Les AS, Shadden SC, Figueroa CA, Park JM, Tedesco MM, Herfkens RJ, Dalman RL, Taylor CA (2010) Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics. Ann Biomed Eng 38:1288–1313. doi:10.1007/s10439-010-9949-x
Les AS, Yeung JJ, Schultz GM, Herfkens RJ, Dalman RL, Taylor CA (2010) Supraceliac and infrarenal aortic flow in patients with abdominal aortic aneurysms: mean flows, waveforms, and allometric scaling relationships. Cardiovasc Eng Technol. doi:10.1007/s13239-010-0004-8
Lesaffre E, Lawson AB (2012) Bayesian Biostatistics
Malek AM, Alper SL, Izumo S (1999) Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042
Martufi G, Auer M, Roy J, Swedenborg J, Sakalihasan N, Panuccio G, Gasser TC (2013) Multidimensional growth measurements of abdominal aortic aneurysms. J Vasc Surg 58:748–755. doi:10.1016/j.jvs.2012.11.070
Metaxa E, Meng H, Kaluvala SR, Szymanski MP, Paluch RA, Kolega J (2008) Nitric oxide-dependent stimulation of endothelial cell proliferation by sustained high flow. Am J Physiol Heart Circ Physiol 295:H736–H742. doi:10.1152/ajpheart.01156.2007
Raghavan ML, Kratzberg J, Castro de Tolosa EM, Hanaoka MM, Walker P, da Silva ES (2006) Regional distribution of wall thickness and failure properties of human abdominal aortic aneurysm. J Biomech 39:3010–3016. doi:10.1016/j.jbiomech.2005.10.021
Ramstack JM, Zuckerman L, Mockros LF (1979) Shear-induced activation of platelets. J Biomech 12:113–125
Rayz VL, Boussel L, Ge L, Leach JR, Martin AJ, Lawton MT, McCulloch C, Saloner D (2010) Flow residence time and regions of intraluminal thrombus deposition in intracranial aneurysms. Ann Biomed Eng 38:3058–3069. doi:10.1007/s10439-010-0065-8
Reich BJ, Hodges JS, Zadnik V (2006) Effects of residual smoothing on the posterior of the fixed effects in disease-mapping models. Biometrics 62:1197–1206. doi:10.1111/j.1541-0420.2006.00617
Salsac AV, Sparks SR, Lasheras JC (2004) Hemodynamic changes occurring during the progressive enlargement of abdominal aortic aneurysms. Ann Vasc Surg 18:14–21. doi:10.1007/s10016-003-0101-3
Silver AE, Vita JA (2006) Shear-stress-mediated arterial remodeling in atherosclerosis: too much of a good thing? Circulation 113:2787–2789. doi:10.1161/CIRCULATIONAHA.106.634378
Stenbaek J, Kalin B, Swedenborg J (2000) Growth of thrombus may be a better predictor of rupture than diameter in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 20:466–469. doi:10.1053/ejvs.2000.1217
Szymanski MP, Metaxa E, Meng H, Kolega J (2008) Endothelial cell layer subjected to impinging flow mimicking the apex of an arterial bifurcation. Ann Biomed Eng 36:1681–1689. doi:10.1007/s10439-008-9540-x
Taylor CA, Hughes TJ, Zarins CK (1999) Effect of exercise on hemodynamic conditions in the abdominal aorta. J Vasc Surg 29:1077–1089
Thubrikar MJ, Labrosse M, Robicsek F, Al-Soudi J, Fowler B (2001) Mechanical properties of abdominal aortic aneurysm wall. J Med Eng Technol 25:133–142
Valant AZ, Ziberna L, Papaharilaou Y, Anayiotos A, Georgiou GC (2011) The influence of temperature on rheological properties of blood mixtures with different volume expanders-implications in numerical arterial hemodynamics simulations. Rheol Acta 50:389–402. doi:10.1007/s00397-010-0518-x
Vorp DA, Lee PC, Wang DH, Makaroun MS, Nemoto EM, Ogawa S, Webster MW (2001) Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg 34:291–299. doi:10.1067/mva.2001.114813
Wang DH, Makaroun MS, Webster MW, Vorp DA (2002) Effect of intraluminal thrombus on wall stress in patient-specific models of abdominal aortic aneurysm. J Vasc Surg 36:598–604
Wurzinger LJ, Opitz R, Blasberg P, Schmid-Schonbein H (1985) Platelet and coagulation parameters following millisecond exposure to laminar shear stress. Thromb Haemost 54:381–386
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31:1116–1128. doi:10.1016/j.neuroimage.2006.01.015
Zambrano BA, Gharahi H, Lim C, Jaberi FA, Choi J, Lee W, Baek S (2016) Association of intraluminal thrombus, hemodynamic forces, and abdominal aortic aneurysm expansion using longitudinal CT images. Ann Biomed Eng 44:1502–1514. doi:10.1007/s10439-015-1461-x
Acknowledgements
Financially supported by the Action “Supporting Postdoctoral Researchers,” co-financed by the European Social Fund (ESF) and the Greek State (LS7_2224). The authors would also like to thank BETA CAE Systems S.A. (Thessaloniki, Greece) for their advice and guidance during the hexahedral meshing using ANSA software.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tzirakis, K., Kamarianakis, Y., Metaxa, E. et al. A robust approach for exploring hemodynamics and thrombus growth associations in abdominal aortic aneurysms. Med Biol Eng Comput 55, 1493–1506 (2017). https://doi.org/10.1007/s11517-016-1610-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-016-1610-x