Abstract
In order to solve the problem of the short lifespan of the neural electrode caused by micro motion, this study designed a novel neural electrode based on lumped compliance compliant mechanism to control different modes of micro-motion in a more effective way. According to the mathematical modeling of the novel neural electrode, the equivalent bending stiffness and equivalent tensile (compression) stiffness were calculated. The results of the finite element analysis based on the mathematical modeling revealed that the novel neural electrode showed excellent micro-motion-attenuation capability. The static analysis results showed that the novel design dramatically reduced the maximum displacement of the brain in 51% and the maximum stress in 41% under longitudinal micro-motion environment. It also effectively reduced the 5.1% maximum stress while maintaining the maximum displacement under lateral micro-motion environment. The experimental results based on the tissue injury evaluation system also confirmed that the novel electrode is more effective in micro-motion attenuation than the reference one. In detail, the strain of the brain tissue caused by the implantation of the neural electrode was decreased by 1.26 to 27.84% at the insertion depth of 3 mm, and 0.522 to 17.24% at the insertion depth of 4.5 mm, which has convinced the effectiveness of the design.

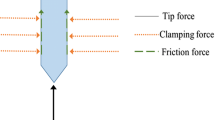
The schematic of the novel neural electrode and evaluationsystem of tissue injury












Similar content being viewed by others
References
Greenhouse I, Gould S, Houser M, Aron AR (2013) Stimulation of contacts in ventral but not dorsal subthalamic nucleus normalizes response switching in Parkinson’s disease. Neuropsychologia 51(7):1302–1309
Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Pahwa R (2009) Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 301(1):63–73
Hohlefeld FU, Ehlen F, Krugel LK, Kühn AA, Curio G, Klostermann F, Nikulin VV (2013) Modulation of cortical neural dynamics during thalamic deep brain stimulation in patients with essential tremor. Neuroreport 24(13):751–756
Patil AC, Thakor NV (2016) Implantable neurotechnologies: a review of micro- and nanoelectrodes for neural recording. Med Biol Eng Comput 54(1):23–44
Minnikanti S, Diao G, Pancrazio JJ, Xie X, Rieth L, Solzbacher F, Peixoto N (2014) Lifetime assessment of atomic-layer-deposited Al 2 O 3–Parylene C bilayer coating for neural interfaces using accelerated age testing and electrochemical characterization. Acta Biomater 10(2):960–967
Sun T, Tsang WM, Park WT, Cheng K, Merugu S (2015) Modeling in vitro neural electrode interface in neural cell culture medium. Microsyst Technol 21(8):1739–1747
Castagnola V, Descamps E, Lecestre A, Dahan L, Remaud J, Nowak LG, Bergaud C (2015) Parylene-based flexible neural probes with PEDOT coated surface for brain stimulation and recording. Biosens Bioelectron 67:450–457
Castagnola E, Ansaldo A, Maggiolini E, Angotzi GN, Skrap M, Ricci D, Fadiga L (2013) Biologically compatible neural interface to safely couple nanocoated electrodes to the surface of the brain. ACS Nano 7(5):3887–3895
Gutowski SM, Templeman KL, South AB, Gaulding JC, Shoemaker JT, LaPlaca MC, Bellamkonda RV, Lyon LA, García AJ (2014) Host response to microgel coatings on neural electrodes implanted in the brain. J Biomed Mater Res A 102(5):1486–1499
Seymour JP, Kipke DR (2007) Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 28(25):3594–3607
Winslow BD, Christensen MB, Yang WK, Solzbacher F, Tresco PA (2010) A comparison of the tissue response to chronically implanted Parylene-C-coated and uncoated planar silicon microelectrode arrays in rat cortex. Biomaterials 31(35):9163–9172
Wu DD, Zhang WG, Merceron G, Luo Y (2013) Mechanical simulation of neural electrode-brain tissue interface under different micro-motion conditions. J Zhejiang Univ 47(2):256–260
Nicolle S, Lounis M, Willinger R, Palierne JF (2005) Shear linear behavior of brain tissue over a large frequency range. Biorheology 42(3):209–223
Streit WJ, Xue QS, Prasad A, Sankar V, Knott E, Dyer A, Reynolds JR, Nishida T, Shaw GP, Sanchez JC (2012) Electrode failure: tissue, electrical, and material responses. IEEE Pulse 3(1):30–33
Fernández E, Greger B, House PA, Aranda I, Botella C, Albisua J, Soto-Sánchez C, Alfaro A, Normann RA (2015) Acute human brain responses to intracortical microelectrode arrays: challenges and future prospects. Front Neuro Eng 7:24
Viventi J, Kim DH, Vigeland L, Frechette ES, Blanco JA, Kim YS, Avrin AE, Tiruvadi VR, Hwang SW, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Spiegel JV, Wu J, Xiao J, Huang Y, Contreras D, Rogers JA, Litt B (2011) Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci 14(12):1599–1605
Lacour SP, Benmerah S, Tarte E, FitzGerald J, Serra J, McMahon S, Fawcett J, Graudejus O, Yu Z, Morrison B (2010) Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces. Med Biol Eng Comput 48(10):945–954
Lind G, Linsmeier CE, Thelin J, Schouenborg J (2010) Gelatine-embedded electrodes—a novel biocompatible vehicle allowing implantation of highly flexible microelectrodes. J Neural Eng 7(4):046005
Lobontiu N (2002) Compliant mechanisms design of flexure hinges. CRC press. Boca Raton.
Zhang W, Ma Y, Li Z (2016) Experimental evaluation of neural probe’s insertion induced injury based on digital image correlation method. Med Phys 43(1):505–512
Nazari MA, Perrier P, Chabanas M, Payan Y (2010) Simulation of dynamic orofacial movements using a constitutive law varying with muscle activation. Comput Method Biomech Biomed Engin 13(4):469–482
Hrapko M, Van Dommelen JA, Peters GW, Wismans JS (2008) The influence of test conditions on characterization of the mechanical properties of brain tissue. J Biomech Eng 130(3):031003
Pinto JT, Touchard F, Castagnet S, Nadot-Martin C, Mellier D (2013) DIC strain measurements at the micro-scale in a semi-crystalline polymer. Exp Mech 53(8):1311–1321
Mesa-Múnera E, Ramírez-Salazar JF, Boulanger P, Branch JW (2012) Inverse-FEM characterization of a brain tissue phantom to simulate compression and indentation. Ingeniería Y Ciencia 8(16):11–36
Acknowledgements
The authors would like to thank Instrumental Analysis Center and Advanced Electronic Materials and Devices Center of Shanghai Jiao Tong University for their generous help.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no.51675330).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, W., Tang, J., Li, Z. et al. A novel neural electrode with micro-motion-attenuation capability based on compliant mechanisms—physical design concepts and evaluations. Med Biol Eng Comput 56, 1911–1923 (2018). https://doi.org/10.1007/s11517-018-1826-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-018-1826-z