Abstract
Zebrafish (Danio rerio) is a powerful animal model used in many areas of genetics and disease research. Despite its advantages for cardiac research, the heartbeat pattern of zebrafish larvae under different stress conditions is not well documented quantitatively. Several effective automated heartbeat detection methods have been developed to reduce the workload for larva heartbeat analysis. However, most require complex experimental setups and necessitate direct observation of the larva heart. In this paper, we propose the Zebrafish Heart Rate Automatic Method (Z-HRAM), which detects and tracks the heartbeats of immobilized, ventrally positioned zebrafish larvae without direct larva heart observation. Z-HRAM tracks localized larva body deformation that is highly correlated with heart movement. Multiresolution dense optical flow-based motion tracking and principal component analysis are used to identify heartbeats. Here, we present results of Z-HRAM on estimating heart rate from video recordings of seizure-induced larvae, which were of low resolution (1024 × 760) and low frame rate (3 to 4 fps). Heartbeats detected from Z-HRAM were shown to correlate reliably with those determined through corresponding electrocardiogram and manual video inspection. We conclude that Z-HRAM is a robust, computationally efficient, and easily applicable tool for studying larva cardiac function in general laboratory conditions.

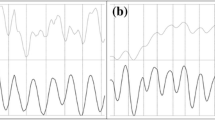
Flowchart of the automatic zebrafish heartbeat detection













Similar content being viewed by others
References
Afrikanova T, Serruys A-SK, Buenafe OEM, Clinckers R, Smolders I, de Witte PAM, Crawford AD, Esguerra CV (2013) Validation of the zebrafish pentylenetetrazol seizure model: locomotor versus electrographic responses to antiepileptic drugs. PLoS One 8:1–8. https://doi.org/10.1371/journal.pone.0054166
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503. https://doi.org/10.1038/nature12111
Lieschke GJ, Currie PD (2007) Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8:353–367. https://doi.org/10.1038/nrg2091
Baraban SC, Taylor MR, Castro PA, Baier H (2005) Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 131:759–768. https://doi.org/10.1016/j.neuroscience.2004.11.031
De Luca E, Zaccaria GM, Hadhoud M et al (2014) ZebraBeat: a flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci Rep 4:1–13. https://doi.org/10.1038/srep04898
Wilkinson RN, Jopling C, van Eeden FJ (2014) Zebrafish as a model of cardiac disease. Prog Mol Biol Transl Sci 124:65–91. https://doi.org/10.1016/B978-0-12-386930-2.00004-5
Singleman C, Holtzman NG (2012) Analysis of post-embryonic heart development and maturation in the zebrafish, Danio rerio. Dev Dyn Off Publ Am Assoc Anat 241:1993–2004. https://doi.org/10.1002/dvdy.23882
Bakkers J (2011) Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc Res 91:279–288. https://doi.org/10.1093/cvr/cvr098
Milan DJ, Jones IL, Ellinor PT, MacRae CA (2006) In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am J Physiol Heart Circ Physiol 291:H269–H273. https://doi.org/10.1152/ajpheart.00960.2005
Chan PK, Lin CC, Cheng SH (2009) Noninvasive technique for measurement of heartbeat regularity in zebrafish (Danio rerio) embryos. BMC Biotechnol 9:1–10. https://doi.org/10.1186/1472-6750-9-11
Pylatiuk C, Sanchez D, Mikut R, Alshut R, Reischl M, Hirth S, Rottbauer W, Just S (2014) Automatic zebrafish heartbeat detection and analysis for zebrafish embryos. Zebrafish 11:379–383. https://doi.org/10.1089/zeb.2014.1002
Balakrishnan G, Durand F, Guttag J (2013) Detecting pulse from head motions in video. IEEE Conf CVPR:3430–3437
Westerfield M (2007) The zebrafish book. University of Oregon Press, Eugene
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310. https://doi.org/10.1002/aja.1002030302
Farnebäck G (2003) Two-frame motion estimation based on polynomial expansion. Proc SCIA’03:363–370
Cho SJ, Byun D, Nam TS et al (2017) Zebrafish as an animal model in epilepsy studies with multichannel EEG recordings. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-03482
Robertson GN, McGee CA, Dumbarton TC et al (2007) Development of the swimbladder and its innervation in the zebrafish, Danio rerio. J Morphol 268:967–985. https://doi.org/10.1002/jmor.10558
Lee SJ, Park SH, Chung JF et al (2017) Homocysteine-induced peripheral microcirculation dysfunction in zebrafish and its attenuation by L-arginine. Oncotarget 8:58264–58271. https://doi.org/10.18632/oncotarget.1681
Pu J, Zheng B, Leader JK, Gur D (2008) An ellipse-fitting based method for efficient registration of breast masses on two mammographic views. Med Phys 35:487–494. https://doi.org/10.1118/1.2828188
Sołtysiński T, Golnik A, Pałko T (2007) Comparison of multiscale entropy of healthy and cancer tissue imaged by optical polarimeter. Pol J Med Phys Eng 13:65–78. https://doi.org/10.2478/v10013-007-0006-5
Bouguet J (2000) Pyramidal implementation of the Lucas Kanade feature tracker. Intel Corp Microprocess Res Labs. Avaliable via CiteSeer. http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.185.585
Sołtysiński T (2006) Bayesian constrained spectral method for segmentation of noisy medical images. Theory and applications. Studies in Computational Intelligence 107:181–206. https://doi.org/10.1007/978-3-540-77662-8_8
Sołtysiński T (2008) Novel quantitative method for spleen’s morphometry in splenomegaly. In: Artificial Intelligence and Soft Computing-ICAISC 2008, vol 5097, pp 981–991. https://doi.org/10.1007/978-3-540-69731-2_93
Sołtysiński T, Kałużynski K, Pałko T (2006) Cardiac ventricle contour reconstruction in ultrasonographic images using Bayesian constrained spectral method. In: Artificial Intelligence and Soft Computing-ICAISC 2006, vol 4029, pp 988–997. https://doi.org/10.1007/11785231_104
Acknowledgements
We would like to thank Cristhian Perez for use of his software in filtering our electrocardiogram data, and Stephan Seo and Neil Jacob for data processing.
Funding
This study was funded by the Foundation for Research of the State of Sao Paulo, Brazil (grant: FAPESP 14/15640-8) and the Brazilian Institute of Neuroscience and Neurotechnology (grant: CEPID-BRAINN 13/07559-3). We are also grateful for the financial assistance provided by the George Mason University Department of Bioengineering and George Mason University Office of Student Scholarship, Creative Activities, and Research (OSCAR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving animals
This article contains studies with animals performed by some of the authors and approved by the Animal Care and Use Committee of University of Campinas, as stated within the manuscript.
Informed consent
No human subjects studies were performed for this manuscript.
Rights and permissions
About this article
Cite this article
Xing, Q., Huynh, V., Parolari, T.G. et al. Zebrafish larvae heartbeat detection from body deformation in low resolution and low frequency video. Med Biol Eng Comput 56, 2353–2365 (2018). https://doi.org/10.1007/s11517-018-1863-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-018-1863-7