Abstract
Differentiation of real interactions between different brain regions from spurious ones has been a challenge in neuroimaging researches. While using electroencephalographic data, those spurious interactions are mostly caused by the volume conduction (VC) effect between the recording sites. In this study, we address the problem by jointly modeling the causal relationships among brain regions and the mixing effects of volume conduction. The VC effect is formulated with a time-invariant linear equation, and the causal relationships between the brain regions are modeled with a nonlinear multivariate autoregressive process. These two models are simultaneously implemented by a multilayer neural network. The internal hidden layers represent the interactions among the regions, while the external layers are devoted for the relationship between the source activities and observed EEG measurements at the scalp. The causal interactions are estimated by the causality coefficient measure, which is based on the information (weights and parameters) embedded in the network. The proposed method is verified using various simulated data. It is then applied to the real EEG signals collected from a memory retrieval test. The results showed that the method is able to eliminate the volume conduction interferences and consequently leads to higher accuracy in identification of true causal interactions.

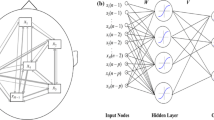
The proposed network structure used to simultaneously model the volume conduction and source interactions. By this special structure, we first move from the sensor space to the source space at the first layer. Then, within internal hidden layers, the interactions between the sources are represented in the form of a general (nonlinear) multivariate autoregressive (nMVAR) model. Finally, we return from the source space to the sensor space at the last layer of the network. The proposed method bypasses the effect of volume conduction and causes more accurate connectivity estimation.







Similar content being viewed by others
References
Kundu B et al (2013) Strengthened effective connectivity underlies transfer of working memory training to tests of short-term memory and attention. J Neurosci 33(20):8705–8715
Bitan T et al (2005) Shifts of effective connectivity within a language network during rhyming and spelling. J Neurosci 25(22):5397–5403
Coben R, Mohammad-Rezazadeh I, Cannon RL (2014) Using quantitative and analytic EEG methods in the understanding of connectivity in autism spectrum disorders: a theory of mixed over-and under-connectivity. Front Hum Neurosci. 8.
Silfverhuth MJ et al (2012) Experimental comparison of connectivity measures with simulated EEG signals. Med Biol Eng Comput 50(7):683–688
Omidvarnia A, et al. (2013) Measuring time-varying information flow for scalp EEG signals: orthogonalized partial directed coherence
Sakkalis V (2011) Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput Biol Med 41(12):1110–1117
Nunez PL et al (1997) EEG coherency: I: statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr Clin Neurophysiol 103(5):499–515
Schoffelen JM, Gross J (2009) Source connectivity analysis with MEG and EEG. Hum Brain Mapp 30(6):1857–1865
Ewald A et al (2012) Estimating true brain connectivity from EEG/MEG data invariant to linear and static transformations in sensor space. NeuroImage 60(1):476–488
Blinowska KJ (2011) Review of the methods of determination of directed connectivity from multichannel data. Med Biol Eng Comput 49(5):521–529
Brunner C et al (2016) Volume Conduction Influences Scalp-Based Connectivity Estimates. Front Comput Neurosci (121):10
Van de Steen F, et al. (2016) Critical comments on eeg sensor space dynamical connectivity analysis. Brain Topogr
Gómez-Herrero G et al (2008) Measuring directional coupling between EEG sources. NeuroImage 43(3):497–508
Michel CM et al (2004) EEG source imaging. Clin Neurophysiol 115(10):2195–2222
Chiang J, Wang ZJ, McKeown MJ (2012) A generalized multivariate autoregressive (GmAR)-based approach for EEG source connectivity analysis. IEEE Trans Signal Process 60(1):453–465
Cheung BL et al (2010) Estimation of cortical connectivity from EEG using state-space models. IEEE Trans Biomed Eng 57(9):2122–2134
McNorgan C, Joanisse MF (2014) A connectionist approach to mapping the human connectome permits simulations of neural activity within an artificial brain. Brain Connect 4(1):40–52
Khadem A, Hossein-Zadeh GA (2014) Estimation of direct nonlinear effective connectivity using information theory and multilayer perceptron. J Neurosci Methods 229:53–67
Farokhzadi M, Hossein-Zadeh G-A, Soltanian-Zadeh H (2018) Nonlinear effective connectivity measure based on adaptive Neuro Fuzzy Inference System and Granger Causality. NeuroImage 181:382–394
Talebi N, Nasrabadi AM, Mohammad-Rezazadeh I (2018) Estimation of effective connectivity using multi-layer perceptron artificial neural network. Cogn Neurodyn 12(1):21–42
Curran T et al (2006) Combined pharmacological and electrophysiological dissociation of familiarity and recollection. J Neurosci 26(7):1979–1985
Benmouiza K, Cheknane A (2013) Forecasting hourly global solar radiation using hybrid k-means and nonlinear autoregressive neural network models. Energy Convers Manag 75:561–569
Babiloni F et al (2005) Estimation of the cortical functional connectivity with the multimodal integration of high-resolution EEG and fMRI data by directed transfer function. Neuroimage 24(1):118–131
Stam CJ (2005) Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol 116(10):2266–2301
Ma H, Aihara K, Chen L (2014) Detecting causality from nonlinear dynamics with short-term time series. Sci Rep 4:7464
Henderson HV, Searle SR (1981) On deriving the inverse of a sum of matrices. SIAM Rev 23(1):53–60
Haufe S et al (2010) Modeling sparse connectivity between underlying brain sources for EEG/MEG. IEEE Trans Biomed Eng 57(8):1954–1963
Schneider T, Neumaier A (2001) Algorithm 808: ARfit—a matlab package for the estimation of parameters and eigenmodes of multivariate autoregressive models. ACM Trans Math Softw 27(1):58–65
Delorme A et al (2011) EEGLAB, SIFT, NFT, BCILAB, and ERICA: new tools for advanced EEG processing. COMPUT INTEL NEUROSC 2011:10–10
Prichard D, Theiler J (1994) Generating surrogate data for time series with several simultaneously measured variables. Phys Rev Lett 73(7):951
Hutcheson NL et al (2015) Effective connectivity during episodic memory retrieval in schizophrenia participants before and after antipsychotic medication. Hum Brain Mapp 36(4):1442–1457
Hampstead BM et al (2011) Activation and effective connectivity changes following explicit-memory training for face–name pairs in patients with mild cognitive impairment: a pilot study. Neurorehabil Neural Repair 25(3):210–222
Gazzaley A, Rissman J, D’esposito M (2004) Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci 4(4):580–599
Ward J (2015) The student’s guide to cognitive neuroscience: Psychology Press.
Danckert S et al (2007) Perirhinal and hippocampal contributions to visual recognition memory can be distinguished from those of occipito-temporal structures based on conscious awareness of prior occurrence. Hippocampus 17(11):1081–1092
Watrous AJ et al (2013) Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat Neurosci 16(3):349–356
Takahashi E, Ohki K, Kim D-S (2007) Dissociated pathways for successful memory retrieval from the human parietal cortex: anatomical and functional connectivity analyses. Cereb Cortex 18(8):1771–1778
Astolfi L et al (2008) Tracking the time-varying cortical connectivity patterns by adaptive multivariate estimators. IEEE Trans Biomed Eng 55(3):902–913
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Talebi, N., Nasrabadi, A.M. & Mohammad-Rezazadeh, I. Bypassing the volume conduction effect by multilayer neural network for effective connectivity estimation. Med Biol Eng Comput 57, 1947–1959 (2019). https://doi.org/10.1007/s11517-019-02006-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-019-02006-w