Abstract
Positron emission tomography (PET) image denoising is a challenging task due to the presence of noise and low spatial resolution compared with other imaging techniques such as magnetic resonance imaging (MRI) and computed tomography (CT). PET image noise can hamper further processing and analysis, such as segmentation and disease screening. The wavelet transform–based techniques have often been proposed for PET image denoising to handle isotropic (smooth details) features. The curvelet transform–based PET image denoising techniques have the ability to handle multi-scale and multi-directional properties such as edges and curves (anisotropic features) as compared with wavelet transform–based denoising techniques. The wavelet denoising method is not optimal for anisotropic features, whereas the curvelet denoising method sometimes has difficulty in handling isotropic features. In order to handle the weaknesses of individual wavelet and curvelet-based methods, the present research proposes an efficient PET image denoising technique based on the combination of wavelet and curvelet transforms, along with a new adaptive threshold selection to threshold the wavelet coefficients in each subband (except last level low pass (LL) residual). The proposed threshold utilizes the advantages of adaptive threshold taken from BayesShrink along with the neighborhood window concept. The present method was tested on both simulated phantom and clinical PET datasets. Experimental results show that our method has achieved better results than the existing methods such as VisuShrink, BayesShrink, NeighShrink, ModineighShrink, curvelet, and an existing wavelet curvelet-based method with respect to different noise measurement metrics, such as mean squared error (MSE), signal-to-noise ratio (SNR), peak signal-to-noise ratio (PSNR), and image quality index (IQI). Furthermore, notable performance is achieved in the case of medical applications such as gray matter segmentation and precise tumor region identification.

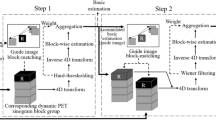
Block diagram of the proposed method. (a) Steps of the proposed denoising method and (b) image information generated by each step of (a).




















Similar content being viewed by others
References
Pieterman RM, van Putten JWG, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koëter GH, Fidler V, Pruim J, Groen HJM (2000) Preoperative staging of non–small-cell lung cancer with positron-emission tomography. England J Med 343(4):254–261
Iwata R, Ido T (1990) Differential diagnosis of lung tumor with positron emission tomography: a prospective
Weber W, Carter Y, Abdel-Dayem HM, Sfakianakis G, et al. (1999) Assessment of pulmonary lesions with (18) f-fluorodeoxyglucose positron imaging using coincidence mode gamma cameras. J Nucl Med 40(4):574
Coxson PG, Huesman RH, Borland L (1997) Consequences of using a simplified kinetic model for dynamic pet data. J Nucl Med 38(4):660
Rodrigues I, Sanches J, Bioucas-Dias J (2008) Denoising of medical images corrupted by poisson noise. In: 15th IEEE International conference on image processing, 2008. ICIP 2008. IEEE, pp 1756–1759
Shih Y-Y, Chen J-C, Liu R-S (2005) Development of wavelet de-noising technique for pet images. Comput Med Imaging Graph 29(4):297–304
Le Pogam A, Hanzouli H, Hatt M, Cheze Le Rest C, Visvikis D (2013) Denoising of pet images by combining wavelets and curvelets for improved preservation of resolution and quantitation. Med Image Anal 17 (8):877–891
Turkheimer FE, Banati RB, Visvikis D, Aston JAD, Gunn RN, Cunningham VJ (2000) Modeling dynamic pet-spect studies in the wavelet domain. J Cerebral Blood Flow Metabol 20(5):879–893
Hannequin P, Mas J (2002) Statistical and heuristic image noise extraction (shine): a new method for processing poisson noise in scintigraphic images. Phys Med Biol 47(24):4329
Seret A, Vanhove C, Defrise M (2009) Resolution improvement and noise reduction in human pinhole spect using a multi-ray approach and the shine method. Nuklearmedizin Nucl Med 48(4):159–165
Ollinger JM, Fessler JA (1997) Positron-emission tomography. IEEE Signal Process Mag 14(1):43–55
Ito K, Xiong K (2000) Gaussian filters for nonlinear filtering problems. IEEE Trans Autom control 45 (5):910–927
Alpert NM, Reilhac A, Chio TC, Selesnick I (2006) Optimization of dynamic measurement of receptor kinetics by wavelet denoising. Neuroimage 30(2):444–451
Candes E, Demanet L, Donoho D, Ying L (2006) Fast discrete curvelet transforms. Multiscale Model Simul 5(3):861–899
Candès EJ, Donoho DL (2004) New tight frames of curvelets and optimal representations of objects with piecewise c2 singularities. Commun Pure Appl Math 57(2):219–266
Ridgelets EJC (1998) Ridgelets: theory and applications. PhD thesis, Ph. D. Thesis, Stanford University USA
Starck J-L, Candès EJ, Donoho DL (2002) The curvelet transform for image denoising. IEEE Trans Image Process 11(6):670–684
Binh NT, Khare A (2010) Multilevel threshold based image denoising in curvelet domain. J Comput Sci Technol 25(3):632–640
Donoho DL, Johnstone IM (1994) Ideal spatial adaptation by wavelet shrinkage. Biometrika, 425–455
Chang SG, Yu B, Vetterli M (2000) Adaptive wavelet thresholding for image denoising and compression. IEEE Trans Image Process 9(9):1532–1546
Shidahara M, Ikoma Y, Kershaw J, Kimura Y, Naganawa M, Watabe H (2007) Pet kinetic analysis: wavelet denoising of dynamic pet data with application to parametric imaging. Ann Nucl Med 21(7):379
Cai TT, Silverman BW (2001) Incorporating information on neighbouring coefficients into wavelet estimation. Sankhyā Indian J Statist, Series B, 127–148
Chen GY, Tien D, Bui, Krzyżak A (2005) Image denoising with neighbour dependency and customized wavelet and threshold. Pattern Recogn 38(1):115–124
Mohideen KS, Perumal AS, Sathik MM (2008) Image de-noising using discrete wavelet transform. Int J Comput Sci Netw Secur 8(1):213–216
Om H, Biswas M (2012) An improved image denoising method based on wavelet thresholding
Green GC (2005) Wavelet-based denoising of cardiac PET data. Carleton University
Taswell C (2000) The what, how, and why of wavelet shrinkage denoising. Comput Sci Eng 2(3):12–19
Mohl B, Wahlberg M, Madsen PT (2003) Ideal spatial adaptation via wavelet shrinkage. J Acoust Soc Am 114:1143–1154
Donoho DL (1995) De-noising by soft-thresholding. IEEE Trans Inf Theory 41(3):613–627
Donoho DL, Johnstone IM (1995) Adapting to unknown smoothness via wavelet shrinkage. J Am Stat Assoc 90(432):1200–1224
Chang GS, Yu B, Vetterli M (1998) Spatially adaptive wavelet thresholding with context modeling for image denoising. In: 1998 International conference on image processing, 1998. ICIP 98. Proceedings, vol 1. IEEE, pp 535–539
AlZubi S, Islam N, Abbod M (2011) Multiresolution analysis using wavelet, ridgelet, and curvelet transforms for medical image segmentation. J Biomed Imag 2011:4
Kumar YK (2009) Comparison of fusion techniques applied to preclinical images: fast discrete curvelet transform using wrapping technique & wavelet transform. J Theor Appl Inf Technol, 5(6)
Ali Hyder S, Sukanesh R (2011) An efficient algorithm for denoising mr and ct images using digital curvelet transform. In: Software tools and algorithms for biological systems. Springer, pp 471–480
Starck J-L, Murtagh F, Fadili JM (2010) Sparse image and signal processing: wavelets, curvelets, morphological diversity. Cambridge University Press
Mallat S (2008) A wavelet tour of signal processing: the sparse way. Academic Press
Wang Z, Bovik AC (2002) A universal image quality index. IEEE Signal Process Lett 9(3):81–84
Slifstein M, Mawlawi O, Laruelle M (2001) Partial volume effect correction: methodological considerations. In: Gjedde A, Hansen SB, GMK, Paulson OB (eds) Physiological imaging of the brain with PET, pp 65–71
Bal A, Banerjee M, Chakrabarti A, Sharma P (2018) Mri brain tumor segmentation and analysis using rough-fuzzy c-means and shape based properties. Journal of King Saud University-Computer and Information Sciences
Bal A, Banerjee M, Sharma P, Maitra M (2018) Brain tumor segmentation on mr image using k-means and fuzzy-possibilistic clustering. In: 2018 2nd International conference on electronics, materials engineering & nano-technology (IEMENTech). IEEE, pp 1–8
Maji P, Pal SK (2011) Rough-fuzzy pattern recognition: applications in bioinformatics and medical imaging, vol 3. Wiley
Kekre HB, Gharge S (2010) Texture based segmentation using statistical properties for mammographic images. Entropy 1:2
Yang H-Y, Wang X-Y, Wang Q-Y, Zhang X-J (2012) Ls-svm based image segmentation using color and texture information. J Vis Commun Image Represent 23(7):1095–1112
Yu S, Muhammed HH (2016) Noise type evaluation in positron emission tomography images. In: International conference on biomedical engineering (IBIOMED). IEEE, pp 1–6
Hasinoff SW (2014) Photon, poisson noise. In: Computer vision. Springer, pp 608–610
Consul PC, Jain GC (1973) A generalization of the poisson distribution. Technometrics 15(4):791–799
Stollnitz EJ, DeRose TD, Salesin DH (1995) Wavelets for computer graphics: a primer part 1 y. Way 6 (2):1
Mulcahy C (1997) Image compression using the haar wavelet transform. Spelman Sci Math J 1(1):22–31
Kara B, Watsuji N (2003) Using wavelets for texture classification. In: IJCI proceedings of international conference on signal processing, vol 1
Candes EJ, Donoho DL (2000) Curvelets, multiresolution representation, and scaling laws. In: International symposium on optical science and technology. International Society for Optics and Photonics, pp 1–12
Donoho DL (2000) Orthonormal ridgelets and linear singularities. SIAM J Math Anal 31(5):1062–1099
Do MN, Vetterli M (2003) The finite ridgelet transform for image representation. IEEE Trans Image Process 12(1):16–28
Candès EJ, Donoho DL (1999) Ridgelets: a key to higher-dimensional intermittency? Philos Trans R Soc London A: Math Phys Eng Sci 357(1760):2495–2509
Acknowledgments
Sincere gratitude to Dr. Punit Sharma, MD at Apollo Gleneagles Hospital, Kolkata, India, for providing the clinical PET brain datasets and verified the results throughout this project. The authors would like to thank Dr. Haseeb Hassan, MD, DM at Rabindranath Tagore International Institute of Cardiac Sciences, Kolkata, India, and Dr. Arindam Chatterjee, MD, at Variable Energy Cyclotron Centre (VECC), Kolkata, India for their helpful comments. The authors would like to thank the referees for providing their very valuable comments on the original version of the manuscript.
Funding
This research work was supported by the Board of Research in Nuclear Sciences (BRNS), DAE, Government of India, under the Reference No. 34/14/13/2016-BRNS/34044.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: Theoretical preliminary
Positron emission tomography plays a vital role in investigating functional analysis in the human brain. The functional activity of PET images should be defined as the distinguishable intensity difference between the objects and their neighborhood. Intensity distribution in the PET image depends on the applied radiopharmaceutical.
1.1 A.1 Noise characteristic in PET image
High noise and poor contrast in PET images can hamper various types of feature recognition processes that are mostly based on the intensity values. Noise characteristics in PET are not well-known till now, so sometimes well-known traditional noise removal techniques cannot perform well in PET image denoising. Typically, it is assumed that noise in PET is characterized as Poisson [5, 9, 10, 44] and mixed Gaussian-Poisson [38]. The effect of Gaussian noise, Poisson noise, and mixed Gaussian-Poisson noise in PET image are shown in Fig. 21 using histograms. For Poisson and mixed Gaussian-Poisson noisy PET image, the intensity scale is distributed with positive values only which are shown in Fig. 21c and d, respectively, whereas, the intensity distribution is seen to have negative values (Fig. 21b) with Gaussian noise which is additive in nature with zero mean and unit deviation. Gaussian noise and Poisson noise are treated as an uncorrelated and correlated component, respectively. Generally, the useful signal is represented as a mean value of that signal, whereas the standard deviation denotes the signal noise.
Gaussian noise
The probability density function of a Gaussian random variable is formulated as
In Eq. 25, i is the intensity level, μ and σ represent mean and standard deviation, respectively. Gaussian distribution is continuous, so a continuous noise can be modeled using the Gaussian distribution.
Let X{Xi,j, i, j = 1,2,…,N} be the noise-free image and Y{Yi,j, i,j = 1,2,…,N} be the noisy image. If the noise type is Gaussian, the noisy image is generated by Eq. 26, where G(i,j) has a normal distribution N(0, 1) and σ is the noise standard deviation.
Poisson noise
The probability density [45] function of a Poisson [46] random variable for a time interval t is formulated as
Here k is a Poisson random variable, e denotes Euler’s number, and the average number of events occurring within an interval is denoted by λ. For Poisson distribution, Eq. 27 is also called probability mass function. In Poisson distribution, the occurrence of each event is independent with respect to other occurrences. A key characteristic of the Poisson distribution is that the variance is the mean. The Poisson distribution is discrete in nature, so a discrete noise can be modeled by using the Poisson distribution.
Gaussian and Poisson noise can be distinguished by the relationship between the mean of pixel intensity and the amplitude of the signal noise which is shown with a simulated phantom in Fig. 22. In the Gaussian noisy image, the amplitude of the Gaussian noise is almost constant throughout the image (Fig. 22e), whereas the amplitude of the Poisson noise is not constant (Fig. 22c) throughout the image because it is proportional to the pixel intensity within its neighborhood region.
Noise effect in simulated Phantom. a Simulated noise-free image with eight homogeneous regions. b Poisson noisy image. c Mean and standard deviation (SD) of Poisson noisy image. d Gaussian noisy image. e Mean and standard deviation of the Gaussian noisy image. In both c and e, 3×3 neighborhood is chosen for measuring the mean and SD of each pixel
1.2 A.2 Wavelet
The wavelet transform [23, 47,48,49] corresponds to the decomposition of a quadratic integral function s(x) 𝜖L2(R) into a family of scaled and translated functions ψk,l(t) is shown in Eq. 28. The functions used in the wavelet transform are localized in the real and Fourier space.
The function ψ(x) is called wavelet function, which describes the band-pass behavior of the signal. The wavelet coefficient (dk,l) is shown in Eq. 29, where ∗ refers to complex conjugate function, k ∈ \(\mathbb {R}\)+ and l ∈ \(\mathbb {R}\).
Wavelet function must be orthogonal to its discrete translation that represents a mathematical condition such as in Eq. 29 called dilation equation where S is treated as a scaling factor.
1.3 A.3 Curvelet
Curvelet transform [50] provides multi-scale and multi-directional transformation with several features compared with wavelets for dealing with directional properties such as edges and curves. Candes et al. [50] decomposed an image into a series of subbands, then applied the concept of random transform and ridgelet transform [14,15,16,17, 51] on each band. The concept of continuous ridgelet function ψa,b,𝜃(y1,y2) was introduced by Cands [14, 50] and formulated as
This is oriented at angles 𝜃 and is constant along the lines y1 cos 𝜃 + y2 sin 𝜃=const. Here, a > 0 is the scale and b is the location. More details of curvelet are described in related Refs. [14,15,16,17, 52]. The presence of ridgelet in the curvelet transform may increase redundancy property. To solve these problems, several researchers [50, 51, 53] redesigned the concept of the curvelet transform to make it simple and easier to implement. The block diagram of the curvelet transform is shown in Fig. 23.
Appendix B: Supplementary data
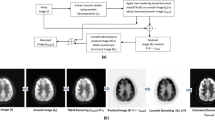
The performance of the different denoising methods for Gaussian and mixed Gaussian-Poisson noisy clinical PET images are shown in Figs. 24 and 25, respectively. The numerical results in Tables 17 and 18 show that the proposed method achieves better results than other denoising methods with respect to MSE, SNR, PSNR, and IQI. The detailed comparative performance analysis of different methods is described in 3.3. The average computation time of different denoising methods for two noisy PET images (Figs. 24 and 25) is shown in Table 19. The detailed analysis of the average computation time is discussed in Section 3.7.
Performance comparison of various denoising techniques on Gaussian noisy (σ = 64) brain PET with noisy PSNR = 33 dB with 3×3 neighborhood window. a Original image. b Noisy image. c VisuShrink [19]. d BayesShrink [20]. e NeighShrink [23]. f ModineighShrink [24]. g Om et al. [25]. h Pogam et al. [7]. i Starck et al. [17]. j Proposed wavelet-curvelet
Performance comparison of various denoising techniques on mixed Gaussian(σ = 80)-Poisson noisy brain PET image with noisy PSNR = 26 dB with 3×3 neighborhood window. a Original image. b noisy image. c VisuShrink [19]. d BayesShrink [20]. e NeighShrink [23]. f ModineighShrink [24]. g Om et al. [25]. h Pogam et al. [7]. i Starck et al. [17]. j Proposed wavelet-curvelet
Rights and permissions
About this article
Cite this article
Bal, A., Banerjee, M., Sharma, P. et al. An efficient wavelet and curvelet-based PET image denoising technique. Med Biol Eng Comput 57, 2567–2598 (2019). https://doi.org/10.1007/s11517-019-02014-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-019-02014-w