Abstract
The aim of this study was the analysis of the mechanical behaviour of a partially porous lumbar custom-made cage by means of a subject-specific finite element analysis (FEA). The cage, made of Ti6Al4V ELI alloy, was produced via electron beam melting (EBM) process and surgically implanted in a female subject, 50 years old. The novelty of this study was the customized design of the cage and of its internal structure, which is impossible to obtain with the traditional production techniques. The 3D model of the spine was obtained from the computed tomography (CT) of the patient. Moreover, high-resolution industrial CT was also used to reconstruct a 3D model of the cage, with its real (as-produced) features, such as superficial roughness, morphology of the bulk and of the porous structure. The workflow was divided in several steps: the main finite element analyses were non-linear and quasi-static regarding: the rhombic dodecahedron (RD) unit cell of the porous structure; the device; the whole L4–L5 motion segment with the implanted cage. Stress distribution was calculated under compression load for all models. For the RD unit cell, the maximum stress appeared at the connected cross nodes, where notch effect was present. For the cage subjected to a load of 1 kN, the porous structure did not present any functional failure. For the whole biomechanical system subjected to a physiological load of 360 N, the calculated stress in the bone was smaller than its yield strength value. On the axial view, a zone with higher compressive stresses was present on the L5 vertebral body. This was due to the contact stress between the cage and the vertebra. From the comparison between FE results and the CT images of the spine, bone remodelling was supposed, with the formation of new bone.

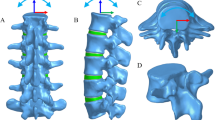
Workflow showing the phases of the research













Similar content being viewed by others
Abbreviations
- FE:
-
Finite element
- FEA:
-
Finite element analysis
- FEM:
-
Finite element method
- RD:
-
Rhombic dodecahedron
- CT:
-
Computed tomography
- EBM:
-
Electron beam melting
- HU:
-
Hounsfield unit
- L5:
-
Lumbar vertebra no. 5
References
de Kunder SL, Rijkers K, Caelers IJMH, de Bie RA, Koehler PJ, van Santbrink H (2018) Lumbar interbody fusion: a historical overview and a future perspective. Spine (Phila Pa 1976) 43:1161–1168. https://doi.org/10.1097/BRS.0000000000002534
Fantigrossi A, Galbusera F, Teresa M et al (2007) Biomechanical analysis of cages for posterior lumbar interbody fusion. 29:101–109. https://doi.org/10.1016/j.medengphy.2006.02.007
Shin DS, Lee K, Kim D (2007) Biomechanical study of lumbar spine with dynamic stabilization device using finite element method. CAD Comput Aided Des 39:559–567. https://doi.org/10.1016/j.cad.2007.03.005
van den Eerenbeemt KD, Ostelo RW, van Royen BJ, Peul WC, van Tulder M (2010) Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur Spine J 19:1262–1280. https://doi.org/10.1007/s00586-010-1445-3
Pimenta L, Oliveira L, Schaffa T, Coutinho E, Marchi L (2011) Lumbar total disc replacement from an extreme lateral approach: clinical experience with a minimum of 2 years’ follow-up. J Neurosurg Spine 14:38–45. https://doi.org/10.3171/2010.9.SPINE09865
Ryan G, Pandit A, Apatsidis DP (2006) Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 27:2651–2670
Sevilla P, Aparicio C, Planell JA, Gil FJ (2007) Comparison of the mechanical properties between tantalum and nickel-titanium foams implant materials for bone ingrowth applications. J Alloys Compd 439:67–73. https://doi.org/10.1016/j.jallcom.2006.08.069
Wen CE, Mabuchi M, Yamada Y et al (2001) Processing of biocompatible porous Ti and Mg. Scr Mater 45:1147–1153. https://doi.org/10.1016/S1359-6462(01)01132-0
Murr LE (2017) Open-cellular metal implant design and fabrication for biomechanical compatibility with bone using electron beam melting. J Mech Behav Biomed Mater 76:164–177. https://doi.org/10.1016/j.jmbbm.2017.02.019
Li SJ, Xu QS, Wang Z, Hou WT, Hao YL, Yang R, Murr LE (2014) Influence of cell shape on mechanical properties of Ti-6Al-4V meshes fabricated by electron beam melting method. Acta Biomater 10:4537–4547. https://doi.org/10.1016/j.actbio.2014.06.010
Zhao S, Li SJ, Hou WT et al (2016) The influence of cell morphology on the compressive fatigue behavior of Ti-6Al-4V meshes fabricated by electron beam melting. J Mech Behav Biomed Mater 59:251–264. https://doi.org/10.1016/j.jmbbm.2016.01.034
Gonzalez Alvarez A, Dearn KD, Shepherd DET (2019) Design and material evaluation for a novel lumbar disc replacement implanted via unilateral transforaminal approach. J Mech Behav Biomed Mater 91:383–390. https://doi.org/10.1016/j.jmbbm.2018.12.011
Tropiano P, Huang RC, Girardi FP et al (2005) Lumbar total disc replacement: seven to eleven-year follow-up. J Bone Jt Surg - Ser A 87:490–496. https://doi.org/10.2106/JBJS.C.01345
Bridgeport DA, Brantley WA, Herman PF (1993) Cobalt-chromium and nickel-chromium alloys for removable prosthodontics, Part 1: Mechanical properties. J Prosthodont 2:144–150. https://doi.org/10.1111/j.1532-849X.1993.tb00398.x
Zhang Z, Li H, Fogel GR et al (2018) Finite element model predicts the biomechanical performance of transforaminal lumbar interbody fusion with various porous additive manufactured cages. Comput Biol Med 95:167–174. https://doi.org/10.1016/j.compbiomed.2018.02.016
Epasto G, Palomba G, D’Andrea D et al (2019) Ti-6Al-4V ELI microlattice structures manufactured by electron beam melting: effect of unit cell dimensions and morphology on mechanical behaviour. Mater Sci Eng A 753:31–41. https://doi.org/10.1016/j.msea.2019.03.014
Akrami M, Qian Z, Zou Z, Howard D, Nester CJ, Ren L (2018) Subject-specific finite element modelling of the human foot complex during walking : sensitivity analysis of material properties , boundary and loading conditions. Biomech Model Mechanobiol 17:559–576. https://doi.org/10.1007/s10237-017-0978-3
Akrami M, Craig K, Dibaj M, Javadi AA (2019) A three-dimensional finite element analysis of the human hip. J Med Eng Technol 0:1–7. https://doi.org/10.1080/03091902.2019.1576795
Jain P, Khan MR (2018) Biomechanical study of fused lumbar spine considering bone degeneracy using FEA. Arab J Sci Eng 43:1325–1334. https://doi.org/10.1007/s13369-017-2848-9
Jain P, Khan MR (2019) Prediction of biomechanical behavior of lumbar vertebrae using a novel semi-rigid stabilization device. Proc Inst Mech Eng Part H J Eng Med 233:849–857. https://doi.org/10.1177/0954411919856497
Haslauer CM, Springer JC, Harrysson OLA et al (2010) In vitro biocompatibility of titanium alloy discs made using direct metal fabrication. Med Eng Phys 32:645–652. https://doi.org/10.1016/j.medengphy.2010.04.003
Li X, Feng YF, Wang CT et al (2012) Evaluation of biological properties of electron beam melted Ti6Al4V implant with biomimetic coating in vitro and in vivo. PLoS One 7:1–12. https://doi.org/10.1371/journal.pone.0052049
Palmquist A, Emanuelsson L, Thomsen P et al (2013) Long-term biocompatibility and osseointegration of electron beam melted, free-form–fabricated solid and porous titanium alloy: experimental studies in sheep. J Biomater Appl 27:1003–1016. https://doi.org/10.1177/0731684411431857
Ponader S, Vairaktaris E, Heinl P et al (2008) Effects of topographical surface modifications of electron beam melted Ti-6Al-4V titanium on human fetal osteoblasts. J Biomed Mater Res - Part A 84:1111–1119. https://doi.org/10.1002/jbm.a.31540
Thomsen P, Malmström J, Emanuelsson L et al (2009) Electron beam-melted, free-form-fabricated titanium alloy implants: material surface characterization and early bone response in rabbits. J Biomed Mater Res - Part B Appl Biomater 90(B):35–44. https://doi.org/10.1002/jbm.b.31250
Schmidt H, Heuer F, Drumm J, Klezl Z, Claes L, Wilke HJ (2007) Application of a calibration method provides more realistic results for a finite element model of a lumbar spinal segment. Clin Biomech 22:377–384. https://doi.org/10.1016/j.clinbiomech.2006.11.008
McBroom RJ, Hayes WC, Edwards WT et al (1985) Prediction of vertebral body compressive fracture using quantitative computed tomography. J Bone Jt Surg Am 67:1206–1214
Morgan EF, Bayraktar HH, Keaveny TM (2003) Trabecular bone modulus-density relationships depend on anatomic site. J Biomech 36:897–904. https://doi.org/10.1016/S0021-9290(03)00071-X
Goel VK, Monroe BT, Gilbertson LG, Brinckmann P (2006) Interlaminar shear stresses and laminae separation in a disc. Spine (Phila Pa 1976) 20:689–698. https://doi.org/10.1097/00007632-199503150-00010
Choi KC, Ryu KS, Lee SH, Kim YH, Lee SJ, Park CK (2013) Biomechanical comparison of anterior lumbar interbody fusion: stand-alone interbody cage versus interbody cage with pedicle screw fixation - a finite element analysis. BMC Musculoskelet Disord 14:1–9. https://doi.org/10.1186/1471-2474-14-220
Zhang QH, Zhou YL, Petit D, Teo EC (2009) Evaluation of load transfer characteristics of a dynamic stabilization device on disc loading under compression. Med Eng Phys 31:533–538. https://doi.org/10.1016/j.medengphy.2008.09.011
Han KS, Rohlmann A, Zander T, Taylor WR (2013) Lumbar spinal loads vary with body height and weight. Med Eng Phys 35:969–977. https://doi.org/10.1016/j.medengphy.2012.09.009
Crupi V, Kara E, Epasto G et al (2017) Static behavior of lattice structures produced via direct metal laser sintering technology. Mater Des:135. https://doi.org/10.1016/j.matdes.2017.09.003
Adams MA, McNally DS, Chinn H, Dolan P (1994) Posture and the compressive strength of the lumbar spine. Clin Biomech 9:5–14. https://doi.org/10.1016/0268-0033(94)90052-3
Sterba M, Aubin CÉ, Wagnac E, Fradet L, Arnoux PJ (2019) Effect of impact velocity and ligament mechanical properties on lumbar spine injuries in posterior-anterior impact loading conditions: a finite element study. Med Biol Eng Comput 57:1381–1392. https://doi.org/10.1007/s11517-019-01964-5
Ominsky MS, Li C, Li X, Tan HL, Lee E, Barrero M, Asuncion FJ, Dwyer D, Han CY, Vlasseros F, Samadfam R, Jolette J, Smith SY, Stolina M, Lacey DL, Simonet WS, Paszty C, Li G, Ke HZ (2011) Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res 26:1012–1021. https://doi.org/10.1002/jbmr.307
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Epasto, G., Distefano, F., Mineo, R. et al. Subject-specific finite element analysis of a lumbar cage produced by electron beam melting. Med Biol Eng Comput 57, 2771–2781 (2019). https://doi.org/10.1007/s11517-019-02078-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-019-02078-8